Scientist Michael T. Pyne shares ARUP’s challenges and process of developing a next generation sequencing (NGS) test for HIV-1 antiretroviral drug resistance.
ARUP Consult, a free source of expert guidance in laboratory testing, has released new and updated resources on testing for immunobullous diseases, diabetes mellitus, and prostate cancer.
ARUP experts are to give six talks and poster presentations at the flagship annual meeting of the American Society for Microbiology (ASM) in Washington, D.C.
ARUP Consult, a free source of expert guidance in laboratory testing, has released new resources on newborn drug screening and noninvasive prenatal testing.
Noninvasive prenatal testing (NIPT) provides critical information to expectant parents. ARUP has updated its test offerings to include the most sensitive and specific alternatives available.
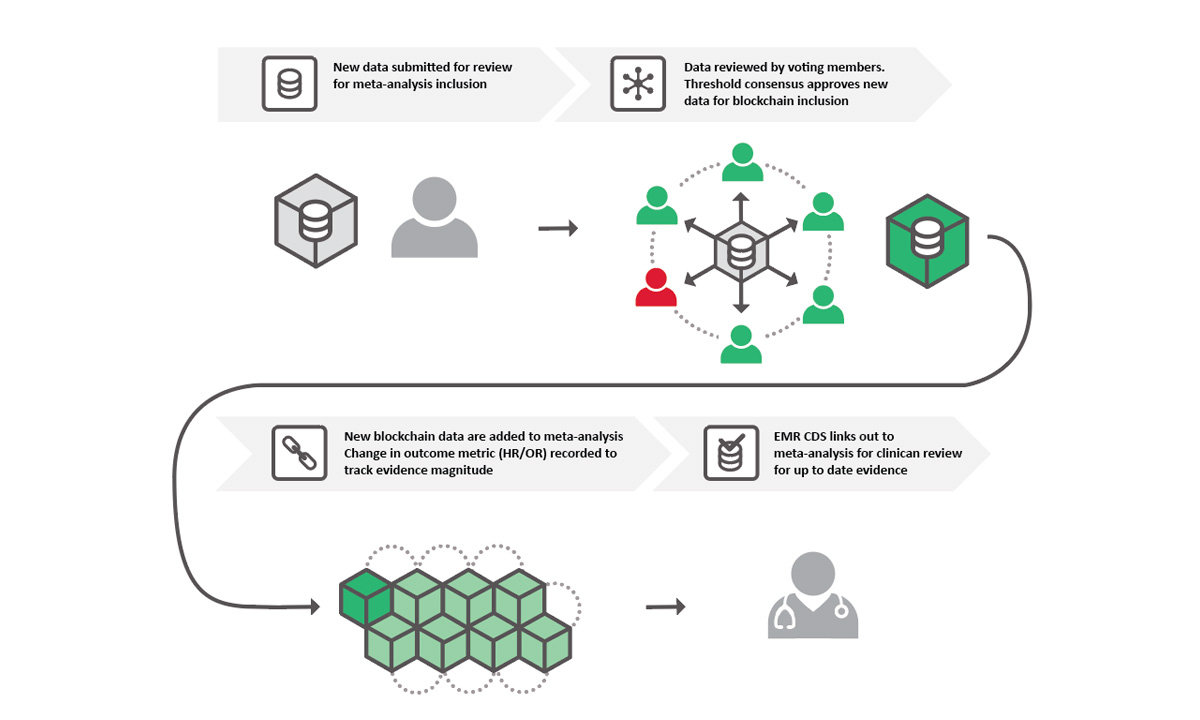
Two members of the ARUP Healthcare Advisory Services team have built the basis for an open access database that could change the way researchers perform meta-analyses.
Society's growing and changing understanding of how race, ethnicity, or ancestry (REA) is experienced and interpreted is prompting many clinical labs to reexamine practices related to REA data.
ARUP study finds pharmacogenetic testing to evaluate drug-gene interactions improves patient outcomes and patients' confidence in overall care.
Biomarker testing can aid in the accurate diagnosis and treatment of cancer, but integrating precision medicine into routine clinical care can be a challenge.
A lot has changed in laboratory medicine during the last 100 years. To celebrate Lab Week 2022, here are five resources that examine the past, present, and future of medical laboratory science.
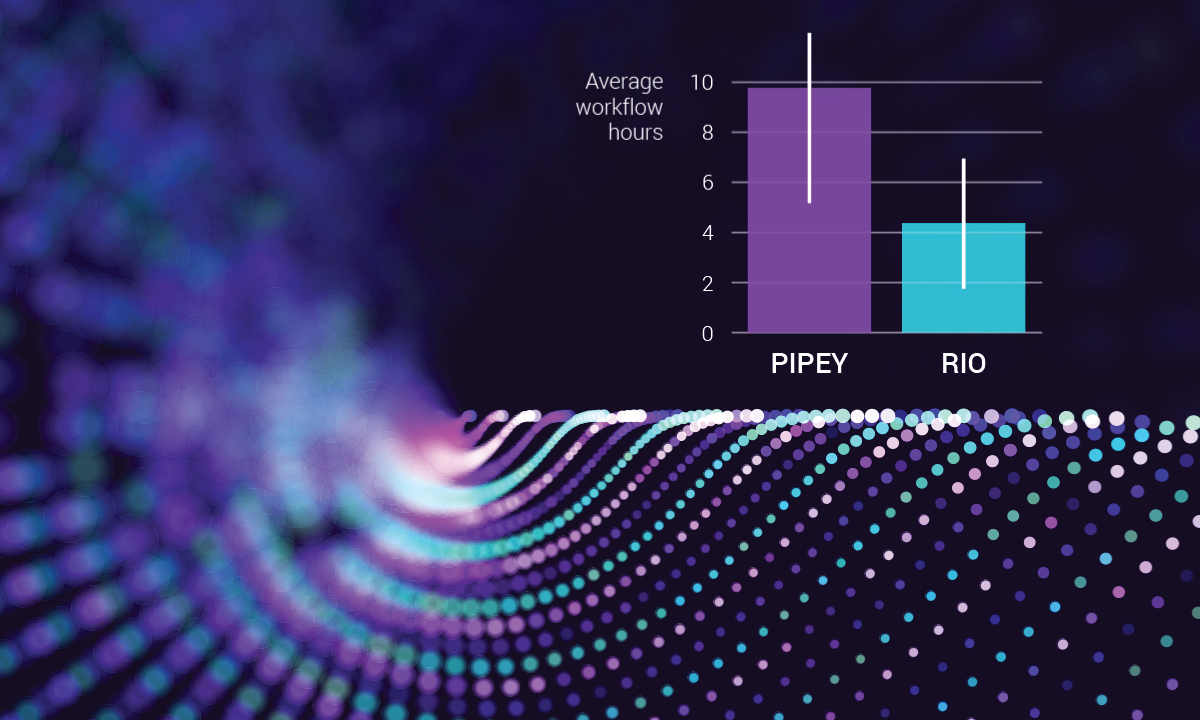
ARUP’s next generation sequencing (NGS) tests are now faster and more precise thanks to the launch of Rio, a new world-class bioinformatics pipeline and analytics platform.
PacBio and ARUP Laboratories announced they are collaborating on a study intended to evaluate whether the solve rate for rare disease cases can be increased.