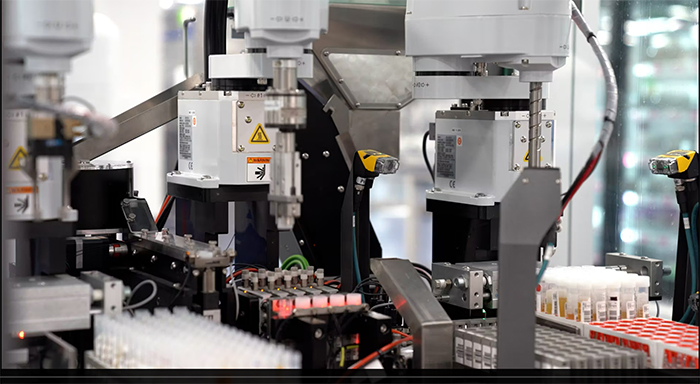
Cutline: ARUP Laboratories is the largest tenant in the University of Utah’s Research Park, occupying 750,000 square feet in eight buildings. ARUP’s main campus includes a 220,000-square-foot, highly automated laboratory facility completed in early 2021.
Photo Credit: ARUP Laboratories
ARUP makes select B-roll footage available for download and use by members of the media in their reporting about ARUP. All videos are copyrighted and must credit ARUP Laboratories.