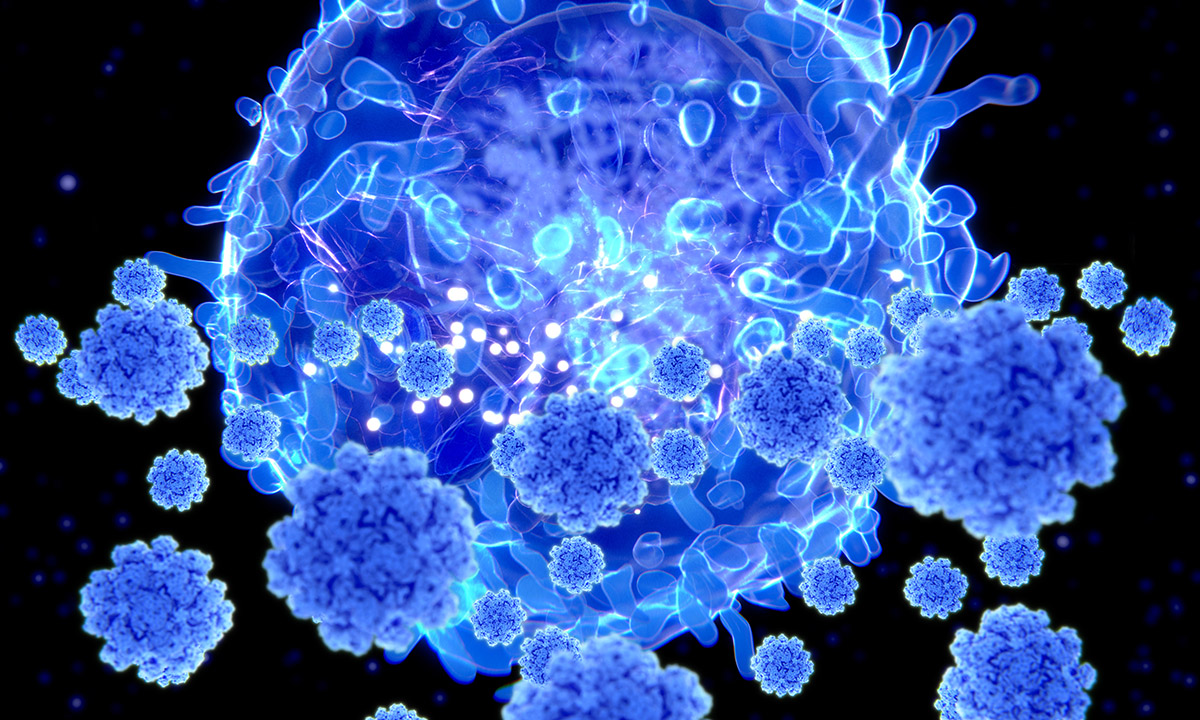
This illustration shows a T lymphocyte cell, or T cell, targeting SARS-CoV-2. When confronted with a new pathogen such as SARS-CoV-2, our immune systems, in addition to developing antibodies, begin over time to call on T cells, a type of white blood cell, not to stop a virus from entering cells as antibodies do, but to prevent the dissemination or propagation of the disease. |
February 2, 2022 |
Research Into T-Cell Response May Hold a Key to Understanding Long-Term Immunity to COVID-19 Two years into the COVID-19 pandemic, when the highly contagious Omicron variant of SARS-CoV-2 has infected millions in the United States alone, most of us have some immunity against the virus. |
|
February 1, 2022 | ARUP Transfusion Medicine has partnered with machine-learning experts at the U of U to develop and validate a method that can predict the need for transfusion during cardiothoracic surgery. |
ARUP’s Employee Advisory Council, pictured here with CEO Andy Theurer and Santa, lead the employee charitable giving program. In 2021, ARUP raised a record $82K for nonprofits that work to cure cancer, improve community health, provide STEM education, prevent suicide, and support those affected by suicide. |
January 20, 2022 |
Topping $82K, Annual Charitable Donations Set New Milestone for ARUP Whatever you call it—altruism, generosity, or simply paying it forward—the act of serving and giving to others is a tenant central to ARUP that goes well beyond the work performed in our labs. |
ARUP Consult, a free source of laboratory testing information for clinicians, released several updated resources in December. |
January 4, 2022 |
ARUP Consult Provides Updated Resources on Testing for Celiac Disease and Trace Elements ARUP Consult®, a free source of expert guidance in laboratory testing, has released updated resources on testing for celiac disease and trace element deficiency and toxicity. |
|
December 17, 2021 | On December 10, 2021, a Common Vulnerability and Exposure (CVE) alert was issued from NIST related to Apache Log4j, CVE-2021-44228. |
|
December 16, 2021 |
ARUP’s Rick Panning: Let’s Fix Reimbursements and Preserve Quality Patient Care Tucked inside the bill that President Biden signed to raise the national debt ceiling was a holiday gift for clinical laboratories and Medicare patients. |