Come to booth #1038 to see ARUP’s showcase of various suites of neurology laboratory testing and meet ARUP medical directors and experts ready to answer questions. |
April 8, 2024 |
ARUP medical directors and experts will be present to discuss the latest developments in |
Tracy George, MD (third from right), ARUP’s chief scientific officer and president of the Innovation Business Unit, holds a zebra, the mascot for patients with rare diseases, and poses for a photo with participants in Salt Lake City’s Rare Disease Day event. Pictured, from left to right, are Tanner Withers, parent of a child with a rare disease, Troy Evans, undiagnosed patient with a rare disease, Rebecca Yates, parent of a child with a rare disease, Matt Pearl, patient with a rare disease, Ava and Lucy Szajunk, undiagnosed patients with rare diseases, George, Chris Gibson, PhD, CEO of Recursion, and Kelvyn Cullimore, president and CEO of BioUtah. |
March 1, 2024 |
ARUP Focuses on Diagnostic Innovation and Supporting Patients on Rare Disease Day ARUP is committed to continue offering quality, esoteric testing that can aid patients with rare diseases on |
MetaCensus is built to support the exchange and review of data by various stakeholders and performs multicriteria decision analysis (MCDA) to assess and account for varying perspectives, counterpoints, and biases. |
February 26, 2024 |
ARUP Experts Develop Tool to Help Reach Scientific Consensus and Speed Advances in Patient Care… MetaCensus is the first open-access data repository built for peer-review and meta- |
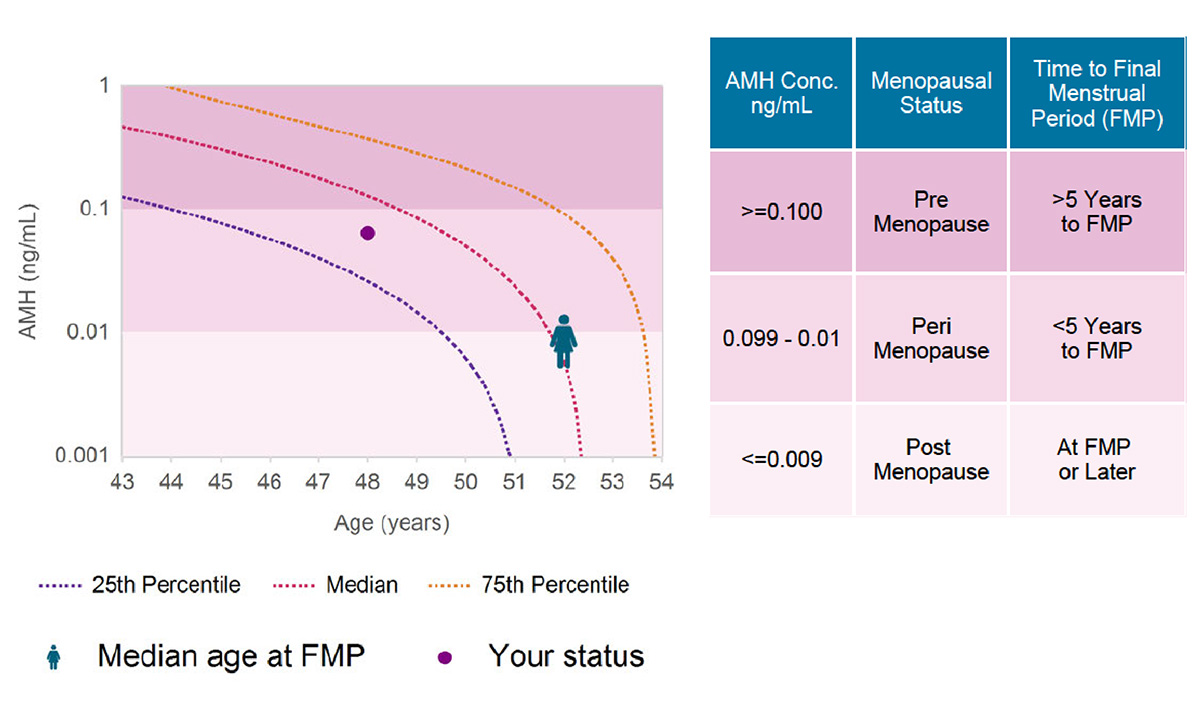
A better estimate of menopausal status will help providers and patients address both the symptoms of menopause and the associated risks, such as cardiovascular disease and osteoporosis. |
February 22, 2024 |
ARUP Offers FDA-Cleared Menopausal Status Test That May Help Women Protect Their Future Health The MenoCheck test helps providers treat women who are experiencing menopausal transition and manage |
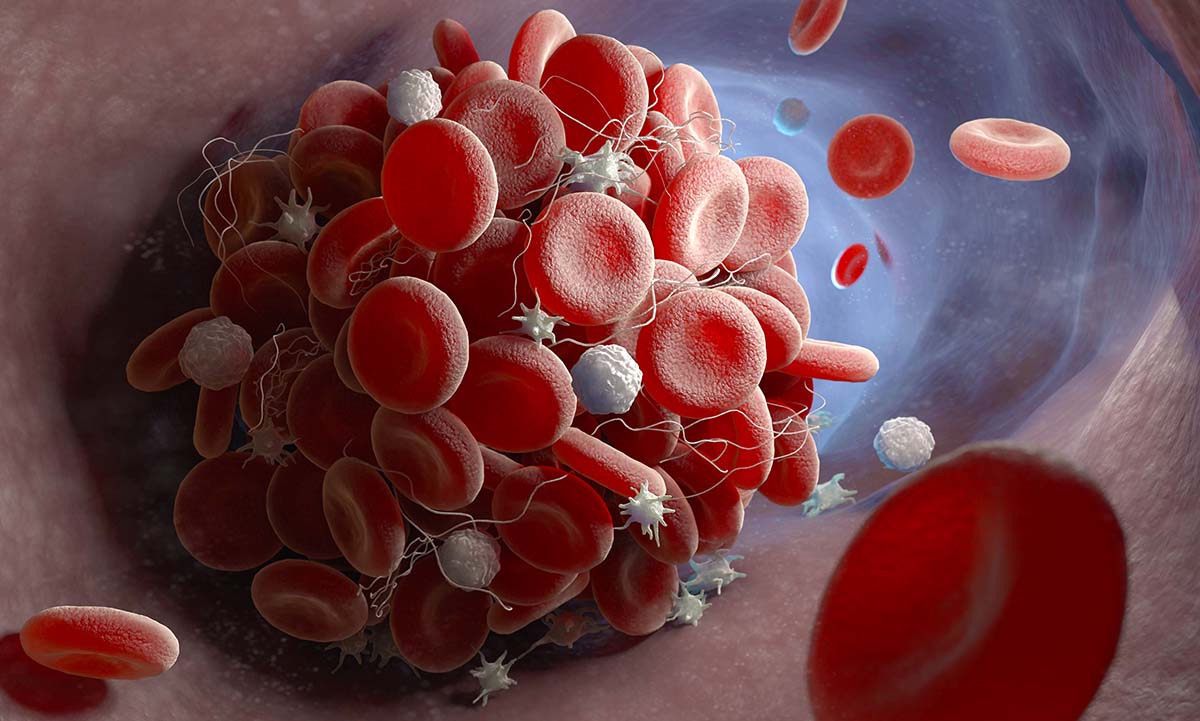
Lupus anticoagulant testing aids in the diagnosis of autoimmune blood clotting disorders. |
February 20, 2024 |
Lupus Anticoagulant Test Neutralizes Interferences to Aid in Diagnosis of Antiphospholipid Syndrome… ARUP’s new lupus anticoagulant panel neutralizes several interfering anticoagulants to |
ARUP medical directors and scientists, including (from left to right) Sherin Shaaban, MD, PhD, FACMG; Yuan Ji, PhD, MBA, DABCP, FACMG; and Hunter Best, PhD, FACMG, along with others, will present their latest research and share insights for current and future geneticists at the Annual Clinical Genetics Meeting in March. |
February 15, 2024 |
ARUP medical directors and scientists will discuss the latest in pharmacogenomics, RNA |