Dan Albertson, MD, will serve as president of the University Business Unit. |
April 1, 2024 |
New ARUP University Business Unit Focuses on Patients and Services at University of Utah Health Dan Albertson, MD, will serve as president of the new University Business Unit and become a member of ARUP’s executive committee. Albertson said service to patients and colleagues will be his focus. |
Michael Pyne, MS, a scientist in ARUP’s R&D Infectious Disease group, performs next generation sequencing (NGS) testing for HIV drug resistance, which is a lab-developed test. |
March 21, 2024 | The FDA’s proposed rule to regulate lab-developed tests will force labs to halt essential testing, harming children and patients with cancer and rare diseases, says ARUP’s Jonathan Genzen, MD, PhD. |
An expert in TAR DNA-binding protein of 43 kDa (TDP-43), neuropathologist Qinwen Mao, MD, PhD, subspecialty director of Neuropathology at ARUP, is working to identify biomarkers that predict the presence of TDP-43 pathology to contribute to the diagnosis of frontotemporal lobar degeneration (FTLD) and Alzheimer’s disease. |
March 20, 2024 |
FDA-Approved Tests and Treatment Offer Hope in the Fight Against Alzheimer’s Disease ARUP’s new test, Alzheimer’s Disease Markers, CSF, will pave the way for more assays that help detect the disease soon enough to try therapy to slow its progression. |
The results of the annual client satisfaction survey show clients’ impressions improved in 2023, building on already positive 2022 results. |
March 15, 2024 |
ARUP’s Customer Service Continues to Rank High in Annual Client Survey Results Of the ARUP clients who participated in the 2023 client satisfaction survey, 93% had a favorable impression of the company, up from 91% in 2022. |
ARUP offers returnships for people from a variety of professional backgrounds who have taken a break from their careers for at least one year. Dipanwita Banerjee, MS, associate scientist, benefited from one such program before joining ARUP. |
March 8, 2024 |
New ARUP Returnships Help Skilled Professionals With an Employment Gap Reenter the Workforce ARUP’s returnships are short-term, paid opportunities designed for skilled professionals with an employment gap of one year or longer for any reason including military service or family obligation. |
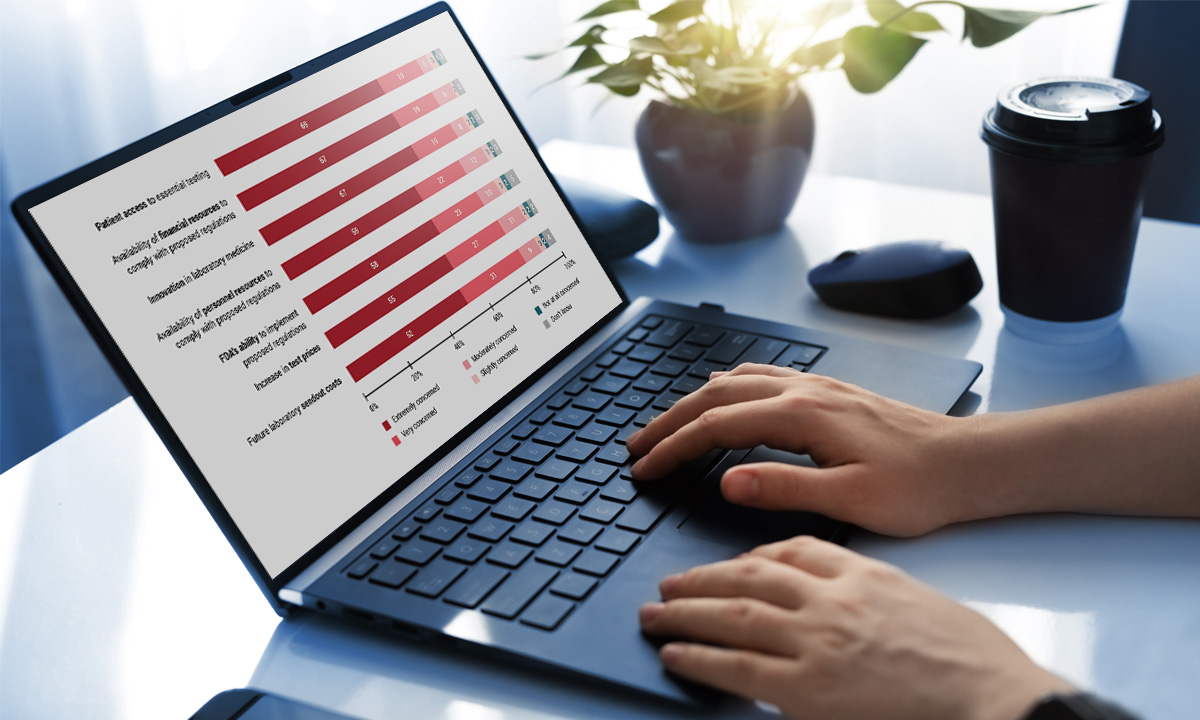
ARUP survey shows “profound” concern within the lab community about the FDA’s proposed rule. |
March 5, 2024 | Nearly 85% of respondents to an ARUP survey believe the FDA’s proposed rule to regulate lab-developed tests will negatively impact their labs. Only 3% believe they have the financial resources to comply. |