Tracy George, MD (third from right), ARUP’s chief scientific officer and president of the Innovation Business Unit, holds a zebra, the mascot for patients with rare diseases, and poses for a photo with participants in Salt Lake City’s Rare Disease Day event. Pictured, from left to right, are Tanner Withers, parent of a child with a rare disease, Troy Evans, undiagnosed patient with a rare disease, Rebecca Yates, parent of a child with a rare disease, Matt Pearl, patient with a rare disease, Ava and Lucy Szajunk, undiagnosed patients with rare diseases, George, Chris Gibson, PhD, CEO of Recursion, and Kelvyn Cullimore, president and CEO of BioUtah. |
March 1, 2024 |
ARUP Focuses on Diagnostic Innovation and Supporting Patients on Rare Disease Day ARUP is committed to continue offering quality, esoteric testing that can aid patients with rare diseases on their often difficult diagnostic journeys. |
Accurate diagnosis and treatment of cardiovascular conditions are key to patient health. ARUP Consult® offers current resources for testing to aid clinicians. |
February 29, 2024 |
ARUP Consult Provides Current Resources on Testing for Cardiovascular Conditions February is American Heart Month, and ARUP Consult has current resources on testing for acute coronary syndrome, heart failure, and atherosclerotic cardiovascular disease markers. |
MetaCensus is built to support the exchange and review of data by various stakeholders and performs multicriteria decision analysis (MCDA) to assess and account for varying perspectives, counterpoints, and biases. |
February 26, 2024 |
ARUP Experts Develop Tool to Help Reach Scientific Consensus and Speed Advances in Patient Care MetaCensus is the first open-access data repository built for peer-review and meta-analysis. The tool aims to break down stakeholder silos to quickly achieve scientific consensus. |
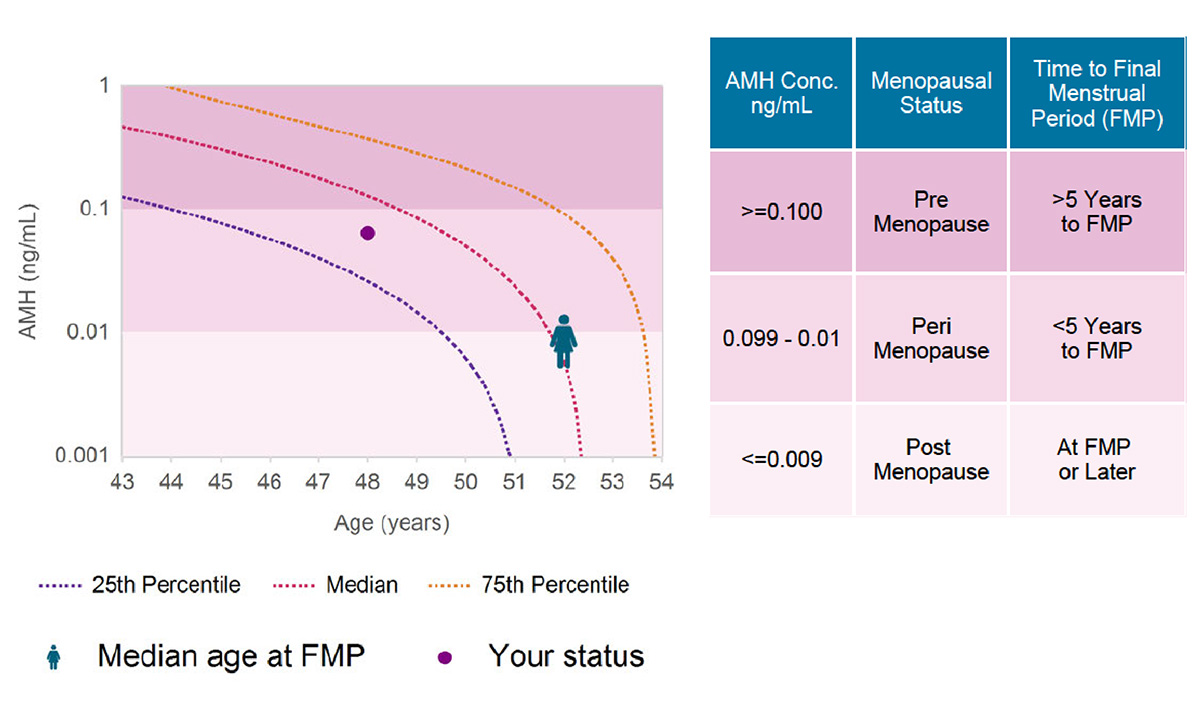
A better estimate of menopausal status will help providers and patients address both the symptoms of menopause and the associated risks, such as cardiovascular disease and osteoporosis. |
February 22, 2024 |
ARUP Offers FDA-Cleared Menopausal Status Test That May Help Women Protect Their Future Health The MenoCheck test helps providers treat women who are experiencing menopausal transition and manage the health risks associated with decreased estrogen production. |
Lupus anticoagulant testing aids in the diagnosis of autoimmune blood clotting disorders. |
February 20, 2024 |
Lupus Anticoagulant Test Neutralizes Interferences to Aid in Diagnosis of Antiphospholipid Syndrome ARUP’s new lupus anticoagulant panel neutralizes several interfering anticoagulants to limit their impact on test results and reduce the likelihood of false-negative and false-positive results. |
ARUP medical directors and scientists, including (from left to right) Sherin Shaaban, MD, PhD, FACMG; Yuan Ji, PhD, MBA, DABCP, FACMG; and Hunter Best, PhD, FACMG, along with others, will present their latest research and share insights for current and future geneticists at the Annual Clinical Genetics Meeting in March. |
February 15, 2024 | ARUP medical directors and scientists will discuss the latest in pharmacogenomics, RNA sequencing, and laboratory genetics career pathways at the upcoming ACMG Annual Clinical Genetics Meeting. |