|
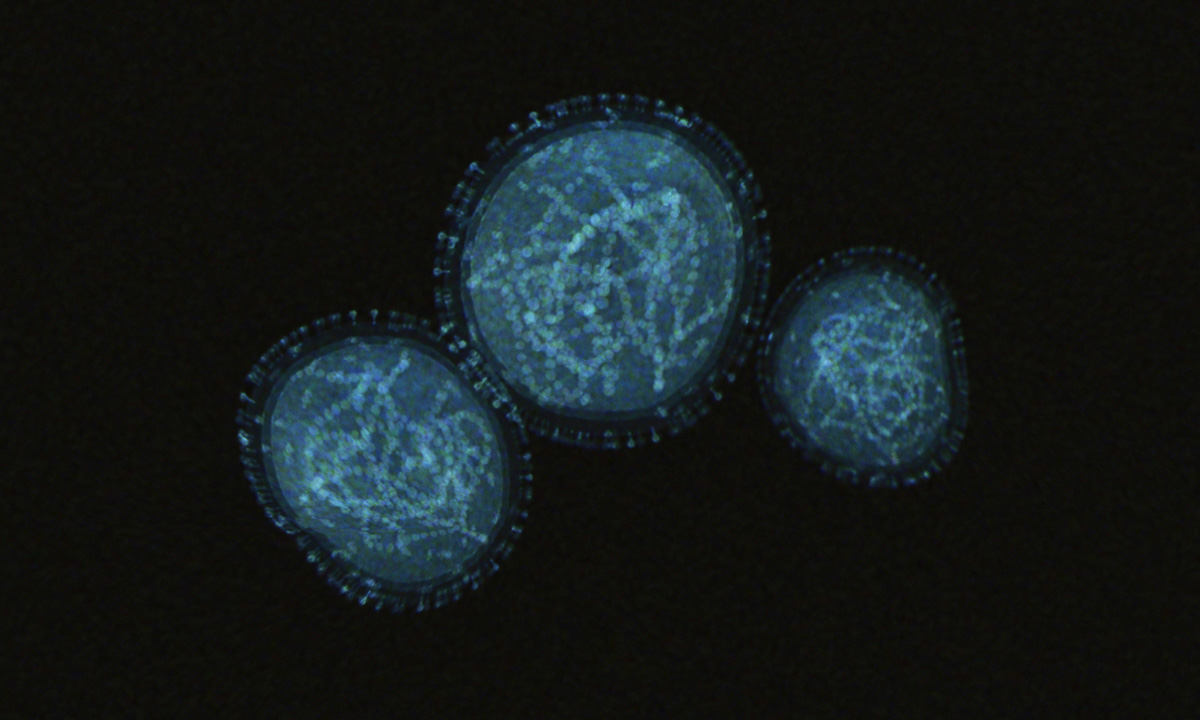
July 25, 2022 | ARUP’s New Monkeypox Test Can Be Performed on More Specimen Types on High-Throughput Instruments ARUP has launched a new, in-house test to detect the DNA of orthopoxviruses, including monkeypox. |
From the left, Tracy George, MD, Jonathan Genzen, MD, PhD, and Adam Barker, PhD, all have gained new responsibilities as part of a strategic alignment of executive responsibilities at ARUP. |
May 9, 2022 | New Executive Appointments Position ARUP Laboratories for Future Growth Jonathan Genzen, MD, PhD, has been named chief medical officer and Adam Barker, PhD, has been named chief operations officer as part of a realignment of executive responsibilities. |
|
April 28, 2022 | ARUP Named to Forbes’ List of America’s Best Employers for Diversity for 2022 ARUP Laboratories’ commitment to creating a supportive and satisfying work environment for employees has earned it a place on Forbes magazine’s list of America’s Best Employers for Diversity. |
|
March 1, 2022 | ARUP Renames Consulting Team as ARUP Healthcare Advisory Services ARUP Laboratories has renamed its expert team of healthcare consultants as ARUP Healthcare Advisory Services. The new name better reflects the broad service offerings the team can provide. |
ARUP’s great culture is getting noticed. Thanks to our dedicated employees for putting us on the Forbes magazine list of America’s Best Midsize Employers for 2022. |
February 11, 2022 | ARUP Laboratories Is Ranked Among America’s Best Midsize Employers by Forbes ARUP’s commitment to creating a satisfying work environment for employees has earned it a spot on Forbes’ list of America’s Best Midsize Employers for 2022. |
|
December 8, 2021 | ARUP Earns Fourth Consecutive Utah Business Best Companies to Work For Award For the fourth consecutive year, Utah Business magazine has honored ARUP Laboratories with its Best Companies to Work For award. ARUP is one of 65 companies to earn the award. |