ARUP is committed to continue offering quality, esoteric testing that can aid patients with rare diseases on their often difficult diagnostic journeys.
February is American Heart Month, and ARUP Consult has current resources on testing for acute coronary syndrome, heart failure, and atherosclerotic cardiovascular disease markers.
MetaCensus is the first open-access data repository built for peer-review and meta-analysis. The tool aims to break down stakeholder silos to quickly achieve scientific consensus.
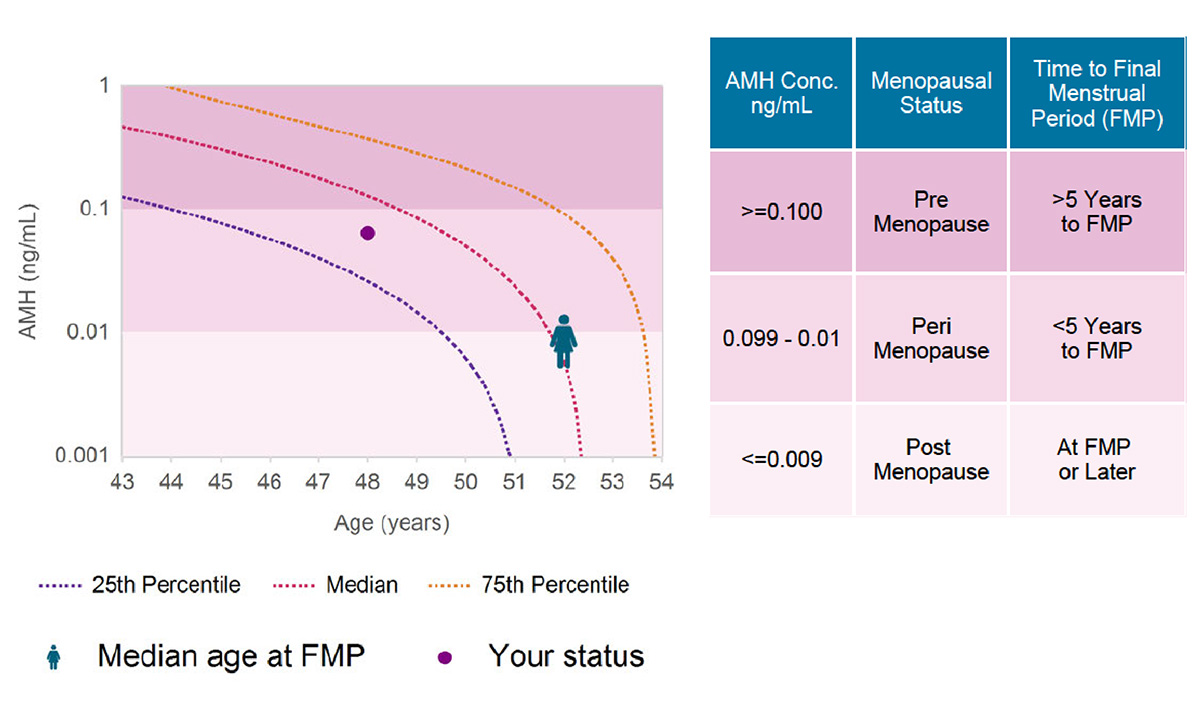
The MenoCheck test helps providers treat women who are experiencing menopausal transition and manage the health risks associated with decreased estrogen production.
ARUP’s new lupus anticoagulant panel neutralizes several interfering anticoagulants to limit their impact on test results and reduce the likelihood of false-negative and false-positive results.
ARUP medical directors and scientists will discuss the latest in pharmacogenomics, RNA sequencing, and laboratory genetics career pathways at the upcoming ACMG Annual Clinical Genetics Meeting.
Researchers at ARUP Laboratories published more than 130 peer-reviewed articles and contributed more than 135 posters or presentations at medical conferences in FY2023 to advance laboratory medicine.
ARUP and the University of Utah Department of Pathology have announced that ARUP’s executive vice president, Julio Delgado, MD, MS, will be the inaugural holder of a new presidential endowed chair.

Four different algorithms to lead clinicians through the correct approach to thyroid testing are available on arupconsult.com.
Lab testing is key to thyroid disease diagnosis and proper treatment but can be challenging. ARUP Consult’s thyroid disease testing resources are divided into easy-to-navigate topics and algorithms.
After wrapping up her education in plant biology, Dipanwita Banerjee, MS, took some time off to see the world and start a family. She later returned to the laboratory environment at ARUP.
Scammers posing as ARUP recruiters on LinkedIn have attempted to collect sensitive information from job seekers. ARUP recommends that potential applicants approach job offers with caution.
Next generation sequencing provides a more rigorous and sensitive method to identify drug-resistant variants of cytomegalovirus, which enables earlier detection and more effective treatment.