Most Popular
February 20, 2025ARUP and other clinical labs anxiously await a decision by a federal court in Texas about whether the FDA overstepped its statutory authority.
April 21, 2025ARUP Laboratories now offers a test for pTau 217 to aid in the detection of Alzheimer’s disease pathology. This test is minimally invasive and more accessible than other types of diagnostic testing.
March 31, 2025The rule and its compliance deadlines are no longer in effect, benefiting patient care, innovation, and the greater laboratory community, ARUP leaders said.
May 30, 2024
ARUP Files Declaration to Support Lawsuit Challenging the FDA's Rule to Regulate Lab-Developed Tests
ARUP’s declaration amplifies the lawsuit’s claims that the FDA does not have legal authority over laboratory testing services and that regulating these services is unreasonable.September 13, 2024ARUP has been awarded a CDC contract for avian influenza A (H5N1) test development, which recognizes our expertise and experience with assay development and our desire to serve public health needs.
January 3, 2025ARUP has launched a new assay for the detection and subtyping of influenza A (H5) virus, which will expand the national capacity to detect the current outbreak of highly pathogenic avian influenza A
March 6, 2025
ARUP Laboratories Expands AI-Augmented Parasitology Screening Tool, Improves Detection and Diagnosis
ARUP will expand its AI-augmented screening tool for the detection of human gastrointestinal parasites to become first and only lab to apply AI to the entire ova and parasite testing process.July 12, 2024ARUP’s chief medical officer urged the House Committee on Ways and Means to consider oversight structures that both protect the public health and support innovation in healthcare.
February 10, 2025Fallon Williams and her daughter have similar heart conditions, which do not have a known genetic cause. Williams hopes whole genome sequencing can provide answers and improve cardiac care for all.
August 27, 2024
ARUP Medical Laboratory Scientist Celebrates More Than 40 Years With University of Utah Hospital Lab
Susan Driggs, a medical laboratory scientist in ARUP’s University of Utah Hospital Clinical Laboratory, has worked in the same lab since before ARUP’s inception in 1984.August 21, 2024A new, first-of-its-kind test uses blood-based biomarkers to assess the risk of developing preeclampsia with severe features and facilitates appropriate interventions.
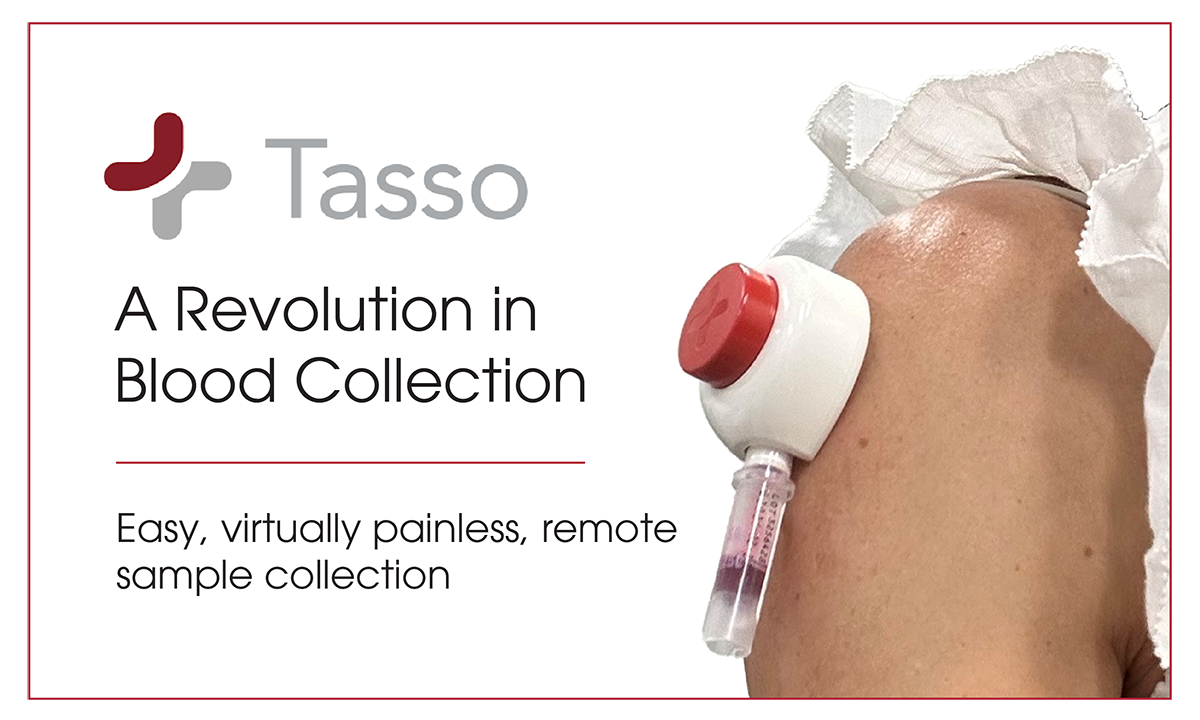
February 3, 2025The partnership combines Tasso’s clinical grade blood collection solutions and ARUP’s gold standard testing capabilities to streamline and accelerate decentralized clinical research.
June 25, 2024Karen Brisendine, a technologist in Mycology AFB, started working at ARUP in 1984 at the company’s founding. In the past 40 years, she has explored many positions, interests, and ARUP departments.
July 8, 2024ARUP Laboratories has been chosen as the site for a House Ways and Means Committee Field Hearing on July 12. Topics include medical innovation, healthcare access, and economic prosperity.
March 17, 2025A comprehensive and detailed article by ARUP experts gives those responsible for anatomic pathology testing a resource for pertinent regulatory information to ensure compliance.
May 28, 2024ARUP Laboratories is committed to helping clients and the clinical laboratory community navigate regulatory changes and is offering current resources and a new webinar.
July 30, 2024The new Sherrie Perkins Research and Innovation Collaboration Grant, designed to foster collaboration in healthcare, will fund cutting-edge research in lab medicine that could impact patient care.
August 16, 2024
ARUP Medical Director Receives Remote and Austere Conditions (RAC) Grant From the University of Utah
Benjamin T. Bradley, MD, PhD, was awarded a grant to study the use of a self-collection blood sample device to increase access to hepatitis C virus testing in rural Utah counties.