Dan Albertson, MD, will serve as president of the new University Business Unit and become a member of ARUP’s executive committee. Albertson said service to patients and colleagues will be his focus.
The FDA’s proposed rule to regulate lab-developed tests will force labs to halt essential testing, harming children and patients with cancer and rare diseases, says ARUP’s Jonathan Genzen, MD, PhD.
ARUP’s new test, Alzheimer’s Disease Markers, CSF, will pave the way for more assays that help detect the disease soon enough to try therapy to slow its progression.
Of the ARUP clients who participated in the 2023 client satisfaction survey, 93% had a favorable impression of the company, up from 91% in 2022.
ARUP’s returnships are short-term, paid opportunities designed for skilled professionals with an employment gap of one year or longer for any reason including military service or family obligation.
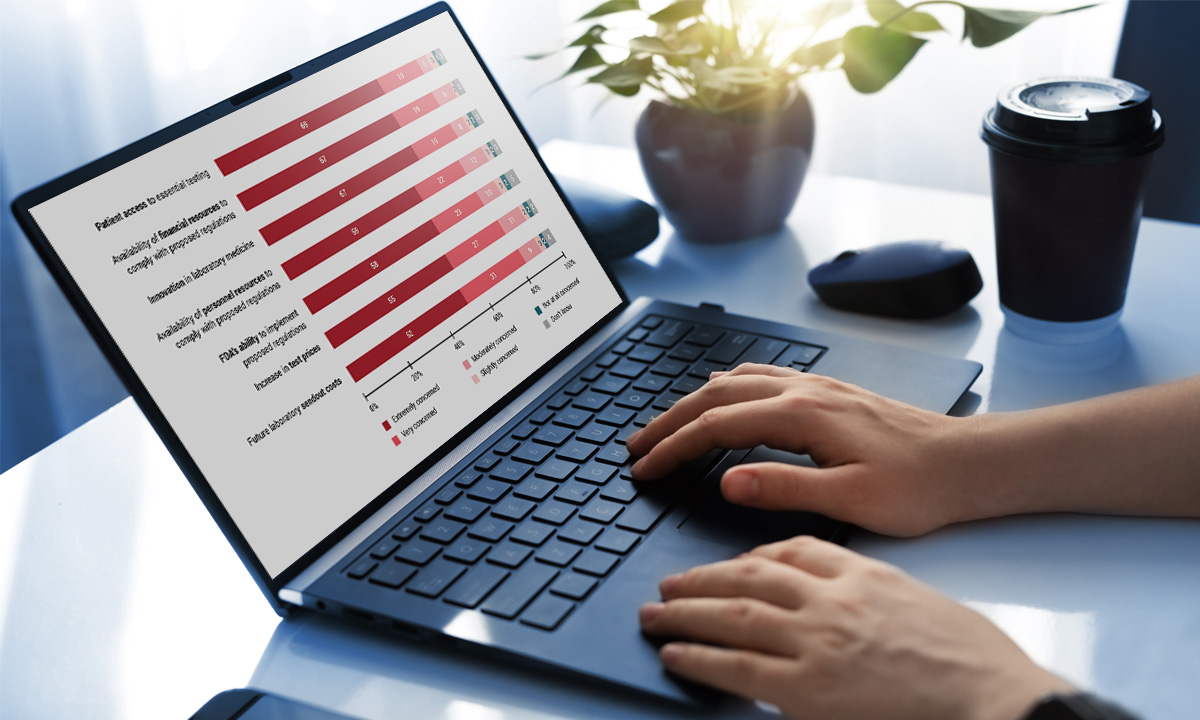
Nearly 85% of respondents to an ARUP survey believe the FDA’s proposed rule to regulate lab-developed tests will negatively impact their labs. Only 3% believe they have the financial resources to…
ARUP is committed to continue offering quality, esoteric testing that can aid patients with rare diseases on their often difficult diagnostic journeys.
February is American Heart Month, and ARUP Consult has current resources on testing for acute coronary syndrome, heart failure, and atherosclerotic cardiovascular disease markers.
MetaCensus is the first open-access data repository built for peer-review and meta-analysis. The tool aims to break down stakeholder silos to quickly achieve scientific consensus.
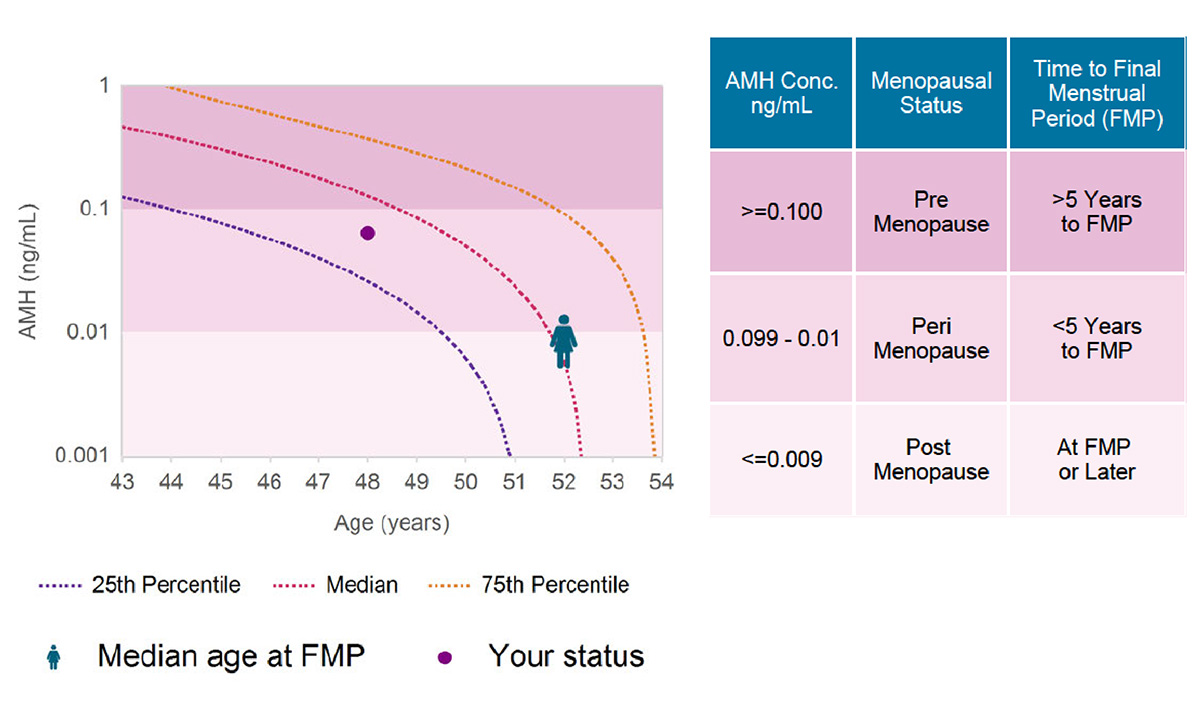
The MenoCheck test helps providers treat women who are experiencing menopausal transition and manage the health risks associated with decreased estrogen production.
ARUP’s new lupus anticoagulant panel neutralizes several interfering anticoagulants to limit their impact on test results and reduce the likelihood of false-negative and false-positive results.
ARUP medical directors and scientists will discuss the latest in pharmacogenomics, RNA sequencing, and laboratory genetics career pathways at the upcoming ACMG Annual Clinical Genetics Meeting.