ARUP Laboratories and Augurex Life Sciences Corp. are partnering to incorporate a 14-3-3η test that will aid in the early detection and management of rheumatoid arthritis. |
October 21, 2024 | Augurex Announces Agreement With ARUP To Expand Access to Advanced Rheumatoid Arthritis Diagnosis ARUP Laboratories is partnering with Augurex Life Sciences Corp. to include the 14-3-3η test in the ARUP test menu to enhance the early detection of rheumatoid arthritis. |
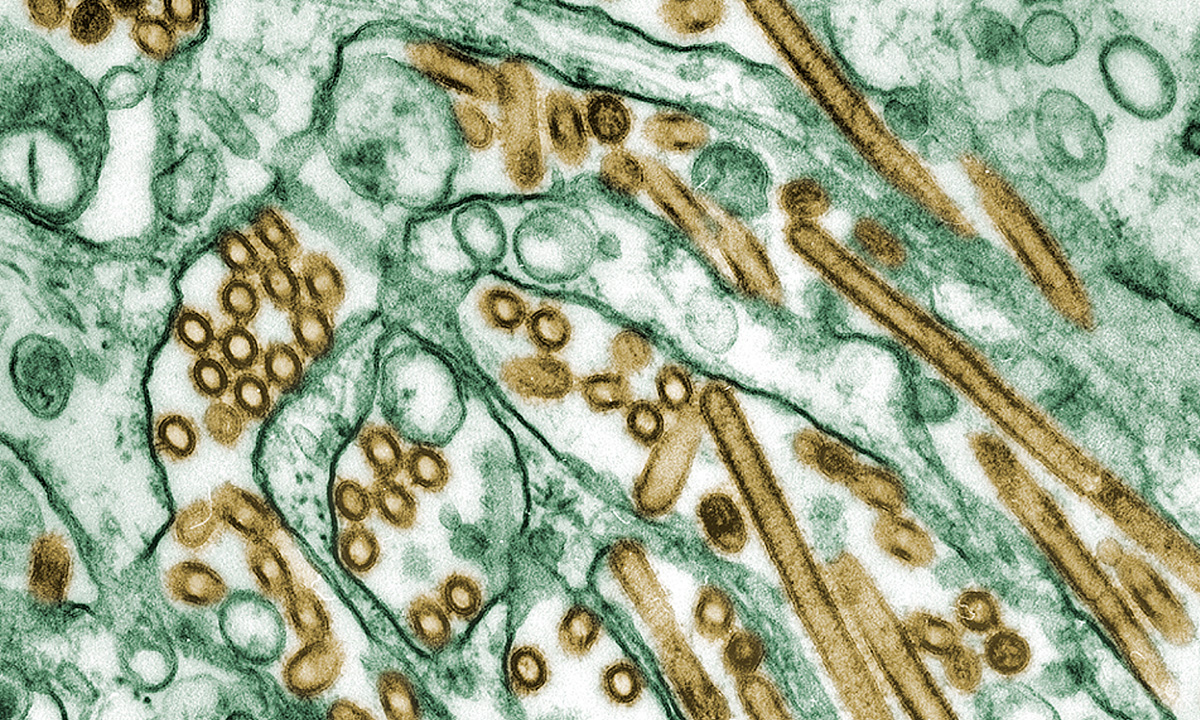
Digitally colorized transmission electron microscopic image of avian influenza A (H5N1) virus particles (in gold), grown in Madin-Darby canine kidney (MDCK) epithelial cells (in green). |
September 13, 2024 | ARUP Awarded CDC Contract for Bird Flu Test Development ARUP has been awarded a CDC contract for avian influenza A (H5N1) test development, which recognizes our expertise and experience with assay development and our desire to serve public health needs. |
The research grant is named in honor of retired ARUP CEO Sherrie Perkins, MD, PhD, whose leadership laid the groundwork for ARUP’s increased focus on innovation. |
July 30, 2024 | New ARUP Laboratories’ Grant Will Fund Innovative Research to Advance Laboratory Medicine The new Sherrie Perkins Research and Innovation Collaboration Grant, designed to foster collaboration in healthcare, will fund cutting-edge research in lab medicine that could impact patient care. |
ARUP Laboratories has filed a declaration supporting a lawsuit against the FDA brought by the American Clinical Laboratory Association. |
May 30, 2024 | ARUP Files Declaration to Support Lawsuit Challenging the FDA's Rule to Regulate Lab-Developed Tests ARUP’s declaration amplifies the lawsuit’s claims that the FDA does not have legal authority over laboratory testing services and that regulating these services is unreasonable. |
ARUP maintains that the FDA’s rule will limit access to testing, stifle innovation, and increase healthcare costs. |
April 29, 2024 | ARUP Statement on FDA Final Rule to Regulate Laboratory-Developed Tests as Medical Devices ARUP maintains that the FDA’s rule will limit access to testing, stifle innovation, and increase healthcare costs. |
The connectivity platform LKOrbit streamlines test ordering, reporting, and billing for laboratory outreach operations. |
April 24, 2024 | ARUP Partners With ELLKAY to Bring Connectivity Solutions to Health System Outreach Operations A new partnership between ARUP and connectivity provider ELLKAY aims to give health systems the testing support, guidance, and technology needed to establish or expand lab outreach operations. |