Dan Albertson, MD, will serve as president of the University Business Unit. |
April 1, 2024 | New ARUP University Business Unit Focuses on Patients and Services at University of Utah Health Dan Albertson, MD, will serve as president of the new University Business Unit and become a member of ARUP’s executive committee. Albertson said service to patients and colleagues will be his focus. |
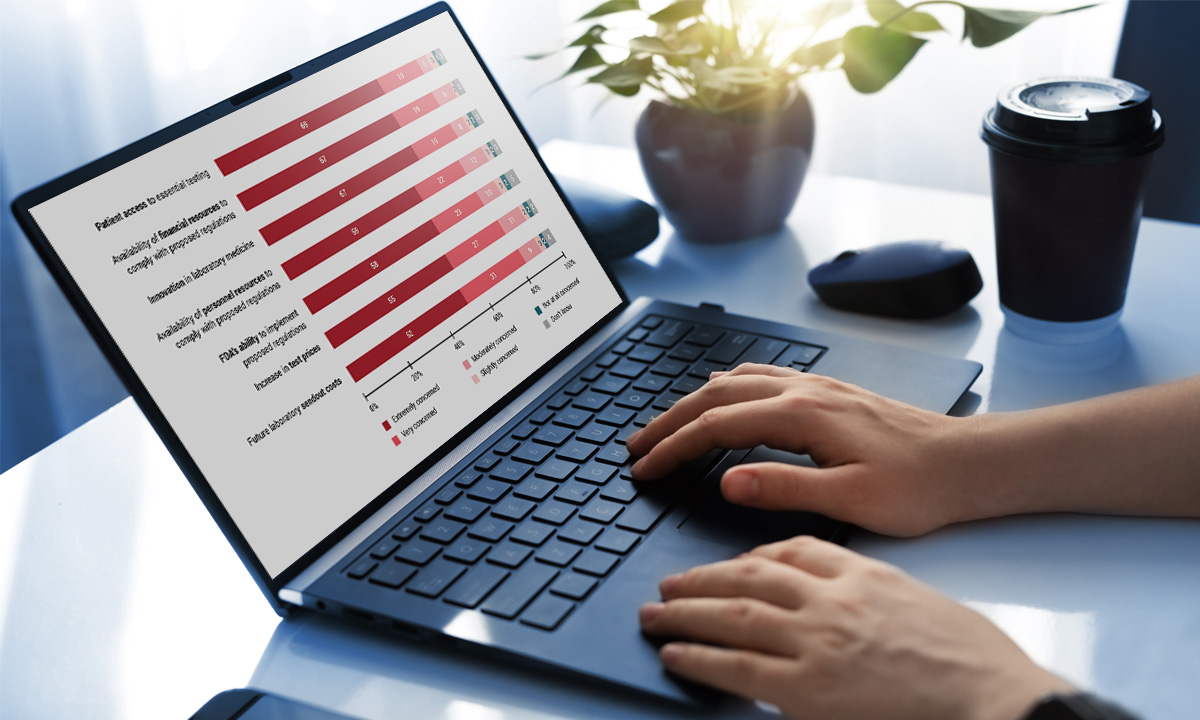
ARUP survey shows “profound” concern within the lab community about the FDA’s proposed rule. |
March 5, 2024 | Lab Community “Extremely Concerned” About Patient Access to Testing If FDA Regulates Lab-Developed Tests Nearly 85% of respondents to an ARUP survey believe the FDA’s proposed rule to regulate lab-developed tests will negatively impact their labs. Only 3% believe they have the financial resources to comply. |
Julio Delgado, MD, MS, ARUP executive vice president and vice chair and chief of Clinical Pathology at the University of Utah, said he is honored to serve as the inaugural Harry R. Hill, MD, Presidential Endowed Chair and continue his mentor’s work in the field of immunology. |
January 25, 2024 | ARUP’s Julio Delgado, MD, MS, Selected as Inaugural Harry R. Hill, MD, Presidential Endowed Chair-Holder ARUP and the University of Utah Department of Pathology have announced that ARUP’s executive vice president, Julio Delgado, MD, MS, will be the inaugural holder of a new presidential endowed chair. |
The AAV5 DetectCDx™ Kit received its Conformitée Européene (CE) mark in January 2022, and testing is being performed by a Medicover facility in Germany. |
December 6, 2023 | Partnership Between ARUP and Medicover Expands Access to Companion Diagnostic in the European Union ARUP and Medicover have partnered to provide a new companion diagnostic test to European Union patients. The test helps identify individuals eligible for a new gene therapy for severe hemophilia A. |
Utah Business magazine has honored ARUP Laboratories with a 2023 Best Companies to Work For Award. ARUP has earned this award every year since 2018. |
December 1, 2023 | ARUP Laboratories Honored With 2023 Best Companies to Work For Award From Utah Business Magazine Utah Business magazine honored ARUP Laboratories with its Best Companies to Work For award for the sixth consecutive year based on anonymous employee survey results. |
ARUP Laboratories has released the company’s public comment in response to the FDA’s plan to regulate LDTs. |
November 29, 2023 | ARUP Laboratories Urges FDA to Withdraw Proposed Rule Regulating Laboratory-Developed Tests ARUP believes the FDA’s plan to regulate lab-developed tests will limit access to testing, increase costs, and face a court challenge, and is calling for collaboration to better address LDT oversight. |