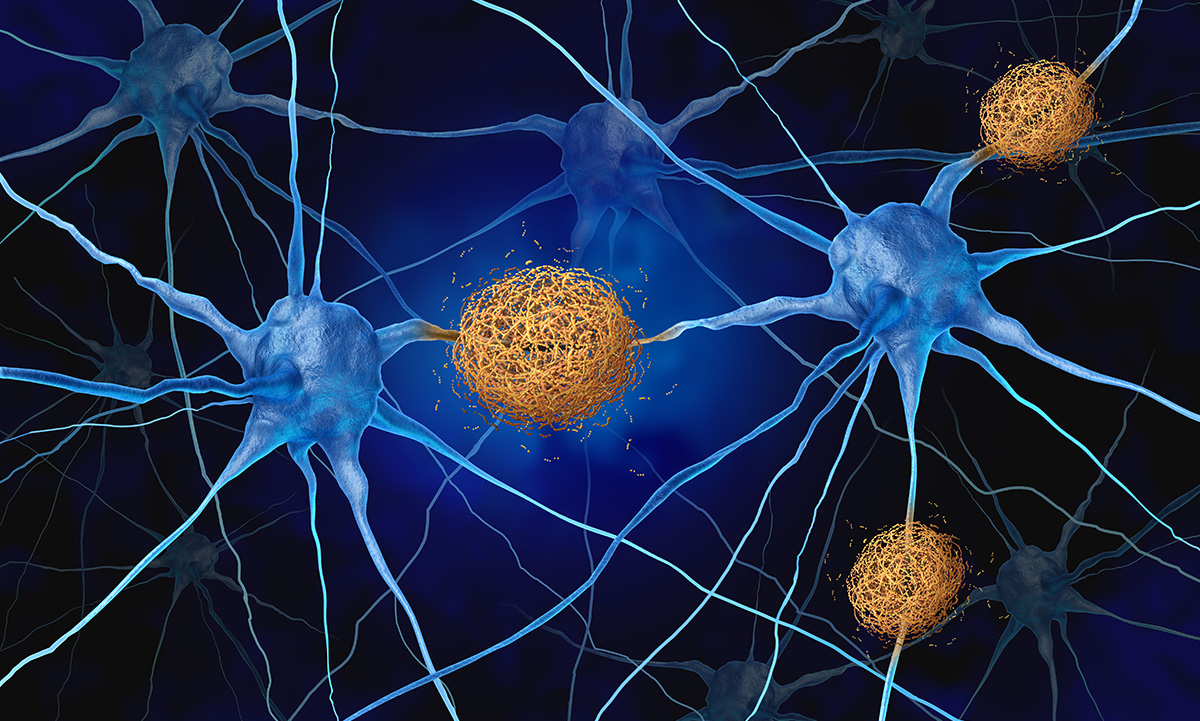
The biomarker pTau 217 is associated with Alzheimer’s disease pathology and can be detected in plasma, providing a minimally invasive option to support other diagnostic tests. |
April 21, 2025 | ARUP Launches pTau 217 Blood Test To Detect Alzheimer’s Disease Pathology ARUP Laboratories now offers a test for pTau 217 to aid in the detection of Alzheimer’s disease pathology. This test is minimally invasive and more accessible than other types of diagnostic testing. |
The U.S. District Court for the Eastern District of Texas today struck down the FDA’s final rule to regulate laboratory-developed tests. |
March 31, 2025 | ARUP Applauds Federal Court Decision Vacating the FDA’s Final Rule Regulating Lab-Developed Tests The rule and its compliance deadlines are no longer in effect, benefiting patient care, innovation, and the greater laboratory community, ARUP leaders said. |
|
March 11, 2025 | Pramana and ARUP Laboratories Partner To Digitize Pathology Slides and Develop AI-Powered Hematopathology Algorithms for Deployment via Edge AI Pramana, Inc., and ARUP are collaborating to improve the assessment of bone marrow biopsies and address other key diagnostic challenges in hematopathology. |
The robotic arm removes the wet-mount specimen slide in preparation for scanning. Once scanned, the image will be screened via artificial intelligence (AI) for evidence of ova and parasites. |
March 6, 2025 | ARUP Laboratories Expands AI-Augmented Parasitology Screening Tool, Improves Detection and Diagnosis ARUP will expand its AI-augmented screening tool for the detection of human gastrointestinal parasites to become first and only lab to apply AI to the entire ova and parasite testing process. |
The partnership combines Tasso’s clinical grade blood collection solutions, such as the Tasso+ device pictured above, with ARUP’s gold standard testing capabilities to streamline and accelerate decentralized clinical research. |
February 3, 2025 | Tasso and ARUP Laboratories Announce Partnership, Combined Offering for Decentralized Clinical Research Biomarker Testing The partnership combines Tasso’s clinical grade blood collection solutions and ARUP’s gold standard testing capabilities to streamline and accelerate decentralized clinical research. |
ARUP’s new assay, Influenza A (H5) Virus by Qualitative NAAT (ARUP test number 3018970), will expand the national capacity to detect the current outbreak of highly pathogenic avian influenza A (H5N1). |
January 3, 2025 | ARUP Offers New Assay To Detect Influenza A (H5) Virus ARUP has launched a new assay for the detection and subtyping of influenza A (H5) virus, which will expand the national capacity to detect the current outbreak of highly pathogenic avian influenza A (H5N1). |