Digitally colorized transmission electron microscopic image of avian influenza A (H5N1) virus particles (in gold), grown in Madin-Darby canine kidney (MDCK) epithelial cells (in green). |
September 13, 2024 | ARUP has been awarded a CDC contract for avian influenza A (H5N1) test development, which recognizes our expertise and experience with assay development and our desire to serve public health needs. |
|
September 4, 2024 | Fraudsters are reportedly posing as ARUP recruiters, offering remote work and sending fake onboarding materials, including fraudulent tax documents, to obtain job seekers’ financial information. |
Ryan Nelson, PharmD, ARUP medical director of Precision Medicine, will moderate a session about standards for pharmacogenetic testing at the Standardizing Laboratory Practices in Pharmacogenomics (STRIPE) Annual Meeting and Consensus Workshop in October. |
August 30, 2024 |
ARUP Medical Director to Moderate Consensus Workshop During STRIPE Annual Meeting Medical Director Ryan Nelson, PharmD, will moderate a STRIPE Annual Meeting workshop in October focused on building consensus around standards for integrating personalized medicine into healthcare. |
Susan Driggs, a medical laboratory scientist in ARUP’s University of Utah Hospital Clinical Laboratory, has worked in the same lab since the 1970s, before ARUP was founded. |
August 27, 2024 |
ARUP Medical Laboratory Scientist Celebrates More Than 40 Years With University of Utah Hospital Lab Susan Driggs, a medical laboratory scientist in ARUP’s University of Utah Hospital Clinical Laboratory, has worked in the same lab since before ARUP’s inception in 1984. |
A new test that ARUP offers for preeclampsia risk measures the ratio between two angiogenic factors (sFlt-1 and PlGF) that are produced by the placenta and are involved in the pathophysiology of preeclampsia. |
August 21, 2024 |
First-of-Its-Kind, FDA-Cleared Test Uses Blood-Based Biomarkers To Assess Risk for Preeclampsia A new, first-of-its-kind test uses blood-based biomarkers to assess the risk of developing preeclampsia with severe features and facilitates appropriate interventions. |
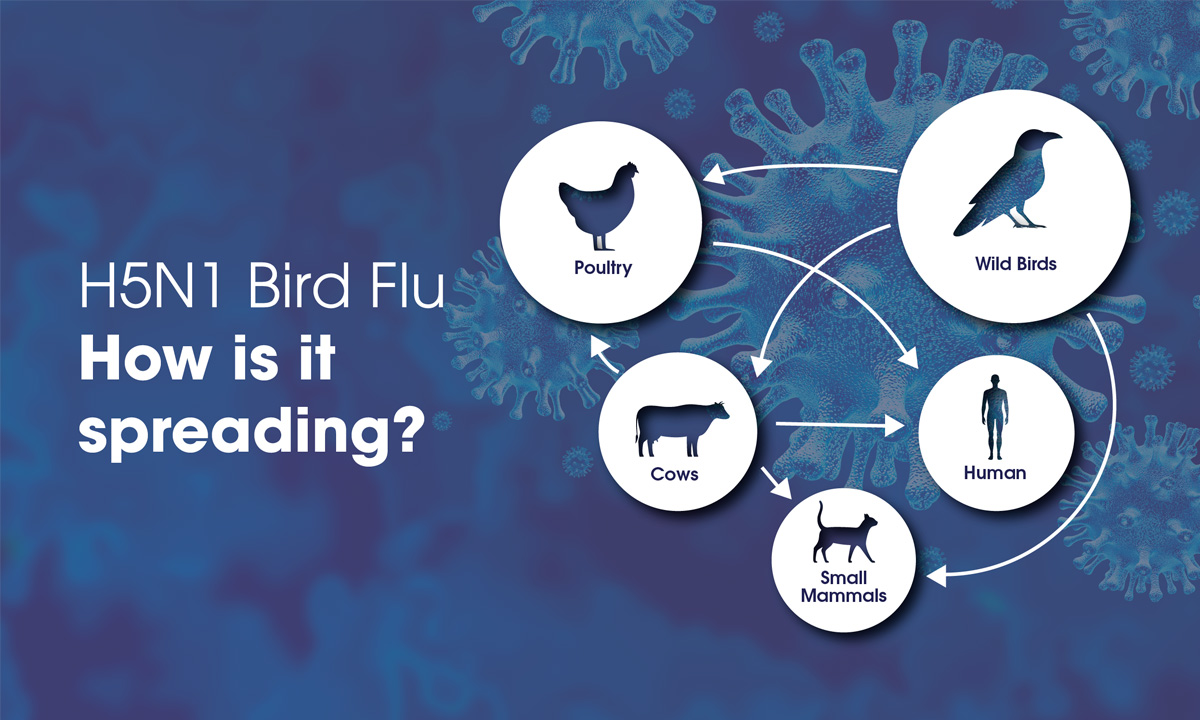
Bird flu is spreading from wild birds to poultry and dairy cows in the United States and in some rare instances has infected humans. |
August 20, 2024 |
ARUP Relies on Experience With Public Health Crises and Prepares a Bird Flu Testing Solution ARUP has been working on a bird flu testing solution for more than a year and is prepared to scale up capacity quickly should the need arise. |