A Federal Court Judge Vacated in Its Entirety the FDA’s Final Rule on Lab-Developed Tests
The rule and its compliance deadlines are no longer in effect, benefiting patient care, innovation, and the greater laboratory community, ARUP leaders said. Read ARUP’s press release.
Access the court’s memorandum opinion and order and final judgment.
Magnify: The Art and Science of Diagnostic Medicine

ARUP’s Message to Clients Amid a Shifting Regulatory Environment: We’ll Continue To Support Our Clients So They Can Support Their Patients
Drawing on more than a decade of experience and engagement in efforts at the federal level, ARUP Laboratories is prepared to support its clients’ needs while also educating and advocating on behalf of all clinical labs as they work to understand the new regulatory environment and its effect on clinical labs.
Read more and watch ARUP leaders discuss the new FDA rule and ARUP’s response in the latest edition of Magnify here.
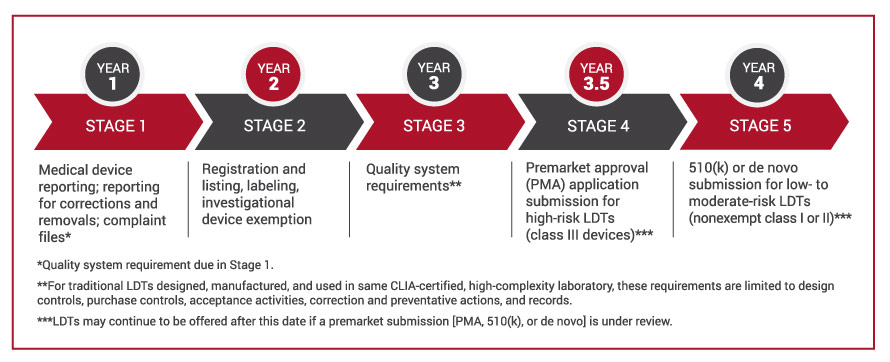
Resource Guides
ARUP Healthcare Advisory Services consultants have compiled a rich set of tools, resources, and templates to help laboratories navigate the requirements that must be met to comply with the FDA’s final rule on laboratory-developed tests (LDTs). Access the guide here.
In addition, ARUP experts have distilled federal laws and regulations in the context of anatomic pathology to create a comprehensive guide available here.
ARUP LabMind Podcast

Jonathan Genzen, MD, PhD, ARUP’s chief medical officer and senior director of governmental affairs, joins Brian Jackson, MD, MS, medical director of Business Development, to answer listeners’ questions on the FDA’s final rule to regulate laboratory-developed tests (LTDs), discuss whether the rule will hold up in court, and explain which tests are subject to which requirements.
ARUP Hosts Informational Webinar on the FDA's Final Rule on LDTs

Summary: The FDA’s final published rule on LDTs will result in new oversight that will dramatically shift how clinical laboratories can develop and offer LDTs going forward. In this recorded webinar, ARUP Chief Medical Officer and Senior Director of Governmental Affairs Jonathan Genzen, MD, PhD, MBA, and Chief Compliance Officer Jonathan Carr, JD, provide an overview of the requirements included in the final rule and how these requirements differ across certain settings and types of testing. The webinar concludes with a Q&A session in which they share their perspectives on questions submitted by registered attendees.
Additional Webinars
Association for Diagnostics and Laboratory Medicine (ADLM) Webinars Featuring Jonathan Genzen, MD, PhD, MBA, ARUP Chief Medical Officer and Senior Director of Governmental Affairs