
FibroMeter is a blood test used to aid in the evaluation and management of liver fibrosis. This test was specifically designed for patients with chronic viral hepatitis (with or without HIV coinfection). This noninvasive blood analysis is from the creators of the FibroScan instrument.
Features and Benefits
- Noninvasive diagnostic test that evaluates the level of fibrosis in the liver from blood biomarkers and patient demographic information
- High diagnostic accuracy confirmed by a rules-based system
- No interference from patients with Gilbert disease or hemolysis (e.g., induced by ribavirin)
- An economic alternative to FibroSURE
- Enhanced graphical reports available
Education Resource
-
Non-invasive Assessment of Liver Fibrosis
by Patricia R. Slev, PhD
Test Details
Scores are algorithmically calculated from values of blood biomarkers, platelet count, and demographic information.
| Measurements Performed by ARUP | Alpha-2-macroglobulin, ALT, AST, GGT, prothrombin index, urea |
|---|---|
| Information Provided by Client | Patient’s platelet count, age, sex |
| Calculated Scores |
Score ranges from 0 to 1, 1 being the most severe stage:
|
| Metavir Classifications |
Corresponding classifications are reported together with the scores:
|
| ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase | |
Comparison between liver biopsy and the noninvasive FibroMeter test:
| FibroMeter | Liver Biopsy | |
|---|---|---|
| Nature of test | Noninvasive | Invasive |
| Advantages | Measurement of global fibrosis, suitable for serial observations | Direct, evaluation of coexisiting pathologies |
| Limitations | Indirect measurement of functional changes of the liver | Sampling error, inter-observer variability, possible hospitalization |
| Risks | Very little risk | Pain, bleeding, pneumothorax, hemothorax, infection |
| Cost | Less expensive than biopsy | Expensive |
| Contradictions | None known | Uncooperative patient, severe coagulopathy, extrahepatic biliary obstruction, ascites, morbid obesity |
Ordering Information
How to Order
Please contact your hospital or reference laboratory to inquire about pricing, test-request forms, and billing for this test.
Test Information
| Test Name | Liver Fibrosis, Chronic Viral Hepatitis (Echosens FibroMeter) |
|---|---|
| Test Code | 2005661 |
| Mnemonic | FIBRO V |
| Method | Quantitative nephelometry, enzymatic, spectrophotometric, and electromagnetic mechanical clot detection |
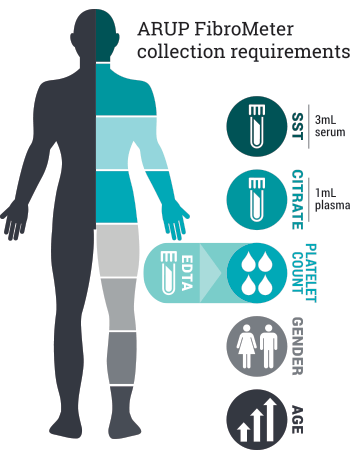
| Specimen Information | 3 mL serum AND 1 mL citrated plasma. Must supply patient platelet count performed on EDTA whole blood (ordered separately through client institution) |
Specimen Collection Requirements
| Collection | Lavender (EDTA) or pink (K2EDTA) tube, serum separator tube, and light blue (sodium citrate) tube |
|---|---|
| Preparation | Separate serum and citrated plasma from cells as soon as possible or within two hours of collection. Transfer 3 mL serum to an ARUP standard transport tube (min: 1.2 mL). Transfer 1 mL platelet-poor citrated plasma to the ARUP standard transport tube (min: 0.5 mL). |
| Transport Temperature | Serum and citrated plasma should be shipped frozen; do not send the EDTA whole blood to ARUP. |
| Remarks | Separately arrange for on-site automated platelet count on the EDTA whole blood sample. Include patient platelet count with test submission information. |
| Specimen Stability |
From the time of collection to initiation of testing:
Serum: refrigerated: unacceptable; frozen: 2 weeks Plasma: refrigerated: unacceptable; frozen: 2 weeks |
Frequently Asked Questions
Does it matter which instrument is used to generate the platelet count?
- No. Many hematology analyzers are available and are suitable for the FibroMeter test.
Why is urea used in FibroMeter?
- Each of the individual analytes in the algorithm have been shown to be a marker for liver function. This includes urea, which decreases with impaired liver function and advanced liver disease. However, the accuracy of using the FibroMeter test to assess fibrosis is based on a proprietary algorithm that evaluates and weighs the concentration of each analyte along with demographic data to provide a score.
Test Interpretation and Strategy
The result states that "FibroMeter and CirrhoMeter scores are modified by the rules-based algorithm." What does this mean?
- To reduce misclassification, test scores are evaluated by a rule-based algorithm to detect anomalous profile results, which may modify test scores as needed.
Can this test be used if a patient has a coinfection of HCV/HIV or HBV/HIV?
- Yes.
Performance and Limitations
Can this test be used on patients receiving ribavirin therapy?
- Yes.
What is the clinical performance of this test?
|
Metavir
Classification |
FibroMeter | |
|---|---|---|
| ≥F2 | F4 | |
| AUROC | 0.85–0.89 | 0.91 |
| Sensitivity (%) | 80.5–89.0 | 94.1 |
| Specificity (%) | 84.1–89.9 | 87.6 |
| PPV (%) | 82.0–86.3 | 68.0 |
| NPV (%) | 77.6–82.5 | 94.7 |
Can this test be run without the platelet count?
- No. The platelet count is necessary for the score calculation. If necessary, platelet count values from an earlier or later draw can be used. However, the date of platelet count must be within three days from the collection of the other blood samples submitted to ARUP for the FibroMeter test.
Can this test be used on a pediatric patient?
- The FibroMeter test was validated on patients age 18 or older. Pediatric specimens will not be rejected, but results should be interpreted with caution.
References
Boursier J et al. Comparison of accuracy of fibrosis degree classifications by liver biopsy and non-invasive tests in chronic hepatitis C. BMC Gastroenterol 2011;11:132.
Calès P, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42(6):1373–81.
Calès P et al. Comparison of liver fibrosis blood tests developed for HCV with new specific tests in HIV/HCV co-infection. J Hepatol 2010;53(2):238–44.
Calès P et al. Evaluating the accuracy and increasing the reliable diagnosis rate of blood tests for liver fibrosis in chronic hepatitis C. Liver Int 2008;28(10):1352–62.
Calès P et al. Improved fibrosis staging by elastometry and blood test in chronic hepatitis C. Liver Int 2014;34(6):907–17.
Imbert-Bismut F, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357(9262):1069–75.
Leroy V, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46(5):775–82.
Leroy V et al. Prospective evaluation of FibroTest®, FibroMeter®, and HepaScore® for staging liver fibrosis in chronic hepatitis B: comparison with hepatitis C. J Hepatol 2014;61(1):28–34.
Sebastiani G et al. Non-invasive assessment of liver fibrosis: it is time for laboratory medicine. Clin Chem Lab Med 2011 49:13-32.
Sebastiani G, et al. Stepwise combination algorithms of non-invasive markers to diagnose significant fibrosis in chronic hepatitis C. J Hepatol. 2006;44(4):686–93.
Shaheen AA, et al. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102(11):2589–600.
















