Thiopurine Drug Toxicity Testing
Questions of Clinical Utility
Thiopurine S-methyltransferase (TPMT) is an enzyme encoded by the TPMT gene that inactivates thiopurine drugs, which suppress the body’s immune system and are used to treat patients with autoimmune disorders, inflammatory bowel disease, and organ transplants.
Changes in the TPMT gene cause TPMT deficiency; without enough of the TPMT enzyme, the body can’t metabolize (turn off) thiopurine drugs. TPMT and xanthine oxidase, an enzyme involved in purine metabolism, work together to inactivate 90 percent of a drug dose, while only 10 percent of the dose is converted to metabolites that stop inflammation and T-cell proliferation.
"The phenotype (enzyme assay) or genotype tests should be performed prior to thiopurine drug therapy to identify patients with abnormal TPMT enzyme activity. Dose adjustments may be required to minimize the risk for toxicity and to optimize therapy.”
Kamisha Johnson-Davis, PhD, DABCC
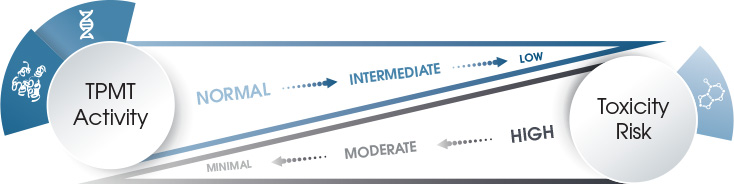
Because xanthine oxidase does not exist in bone marrow, the risk for life-threatening bone marrow toxicity depends on TPMT enzyme activity. Patients with low TPMT activity are at increased risk for thiopurine drug-induced toxicity when treated with a standard drug dose. Patients with high TPMT activity, however, may be undertreated.
Due to single nucleotide polymorphisms (SNPs; pronounced “snips”) in the TPMT gene, TPMT enzyme activity varies within a population. SNPs occur at a specific position in a gene and are the most common genetic variations within a population..
Altering the function of genes encoding drug-metabolizing enzymes can impact how an individual responds to a given drug. For example, there are more than 30 alleles on the TPMT genes, but four of them—*2, *3A, *3B, and *3C—account for approximately 95 percent of low enzyme activity in a specific population.
Patients who inherit two nonfunctional alleles on their opposite chromosomes have an increased risk for severe bone marrow toxicity from conventional doses of thiopurine drugs. The most common functional allele found in the Caucasian population is TPMT*3A. The most common allele variant found in the Asian and African populations is TPMT*3C.
People of Caucasian race follow a tri-modal—low, intermediate, and high—distribution pattern of TPMT enzyme activity. About 89 percent of Caucasians with high TPMT enzyme activity have the homozygous (two of the same allele) wide-type genotype, while 11 percent of those with intermediate enzyme activity have one wild-type and one variant allele. However, one out of every 300 individuals with low enzyme activity has two low TPMT activity alleles or is homozygous for the deficient alleles.
What is the best way to utilize TPMT genotyping or phenotyping tests?
TPMT genotyping or phenotyping should be performed prior to thiopurine administration to predict the risk of developing severe bone marrow toxicity. For the phenotype (enzyme) assay, patients should currently not be on thiopurine therapy, as the substrate for the enzyme assay is 6-mercaptopurine.
If a patient is on thiopurine therapy, the thiopurine drug metabolite assay can be ordered to assess TPMT activity by measuring levels of 6-methyl mercaptopurine (6-MMP) and 6-thioguanine nucleotides (6-TG) nucleotides to determine if the patient has normal metabolism (i.e., metabolites are within the therapeutic reference range).
The genotype assay can also be ordered post thiopurine drug therapy. If a patient is identified as having low or intermediate TPMT activity, dose adjustments should be made to minimize the risk for bone marrow toxicity and optimize therapy.
The Clinical Pharmacogenetics Implementation Consortium (CPIC) has published guidelines for TPMT and thiopurine dosing to guide clinicians in proper dose adjustment based on TPMT genotype and likely phenotype.
What are the limitations of genotype and phenotype testing?

Several drugs can alter or inhibit TPMT enzyme activity, which may lead to falsely low results (e.g., TPMT activity in red blood cells can be masked if the patient has received a recent blood transfusion). Saliva genotype assays have been proposed to overcome this limitation. Conventional TPMT genotyping assays are designed to target the most common TPMT SNPs but don’t allow for the detection of rare alleles. Testing both TPMT enzyme activity and TPMT genotyping can help minimize effects of these limitations, thus greatly enhancing clinical utility.
Several recent studies have reported that individuals who carry low functional alleles for the NUDT15 gene encoding the Nudix hydrolase 15 cannot tolerate standard doses of thiopurine drugs.
Clinicians should keep in mind that TPMT genotype and phenotype tests do not replace the need for careful clinical monitoring.
What is the evidence that genotype and phenotype testing improves patient outcomes?
TPMT pharmacogenetics is one of the first examples that demonstrated the clinical utility of pharmacogenetic testing. Genetically low TPMT activity was first linked to thiopurine drug-associated, life-threatening toxicities in 1989; since then, multiple studies have confirmed this association in a variety of disease settings.
Based on strong and consistent clinical evidences, CPIC has issued guidelines with strong recommendations for considering alternative agents or drastically reducing doses for patients carrying low or deficient TPMT alleles prior to drug therapy.
TPMT gene and related thiopurines drugs (i.e., thioguanine, mercaptopurine, and azathioprine) were also listed in the table of pharmacogenetic biomarkers in drug labeling by the U.S. Food & Drug Administration.

















 HOME
HOME