Whole Genome Sequencing Provides Rapid Diagnosis of Genetic Disorders in Critically Ill Newborns
ARUP Research Partnership With U of U Health Is Transforming Care
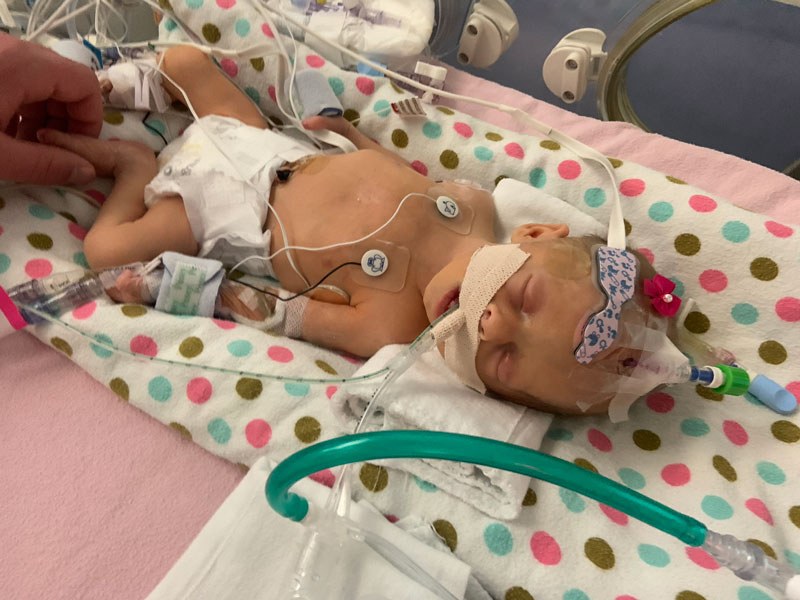
Genetic experts at ARUP Laboratories know her best by her NeoSeq patient characteristics: a 3-day-old girl, born at 34 weeks of gestation with a congenital diaphragmatic hernia (CDH) that pushed her liver, stomach, and bowels up into her chest.
She is a tiny patient with a medical riddle to unravel. Her condition has the potential to change what medical experts know about her genetic disorder.
To her parents, she is just Nevaeh—that’s “heaven” spelled backward—their precious first child. Since her birth on January 5, 2021, she has spent almost 14 months in the University of Utah Hospital’s Neonatal Intensive Care Unit (NICU) facing more serious and complicated medical hurdles than they can count.
“So far, she’s come out on top every time, even when nobody expected it,” said Kimberly Pass, Nevaeh’s mother. “She’s pretty amazing, and she’s taught the people who have been doing this for years.”
Born without a diaphragm to hold her abdominal organs in place, Nevaeh is one of about 30 children enrolled in the Utah NeoSeq Project, a study of rapid whole genome sequencing that is changing the future of neonatal genetic disorder diagnosis.
Advancements in genetic sequencing technology have enabled researchers to simultaneously compare a trio of DNA samples from a baby and both parents to dramatically shorten the time span from birth to diagnosis; instead of weeks or months, diagnoses can be made in roughly 72 hours or less.
That’s a timetable that can dramatically change the course of an infant’s life. The faster the diagnosis, the faster treatment plans can be made, and care can begin, and the sooner families will know what to expect.
“It’s staggering that we are able to do this in the time that we are able to do it,” said Hunter Best, PhD, FACMG, the scientific director of Next Generation Sequencing and Biocomputing, whose team oversees genomic sequencing at ARUP. “This is definitely going to change the face of medicine.”

Launched in early 2020, NeoSeq is a collaboration between ARUP, University of Utah Health, and the California-based sequencer manufacturer Illumina. The project brings together dozens of medical experts, researchers, scientists, and informaticists to draw on their expertise in molecular genetics and genomic medicine to jointly assess patient information and work toward diagnosis.
“That’s the beauty of this project,” said Rong Mao, MD, FACMG, medical director of Molecular Genetics and Genomics at ARUP. “We are not just alone in the laboratory; we are working together with NICU physicians and scientists as we review cases. Everyone is learning through the process.”

In the simplest terms, genetic sequencing has two primary steps. The first step uses sequencers in the ARUP Genomics Lab to generate data from the approximately 3 billion base pairs of DNA in the human genome. The goal is to sift out uninformative genetic information and identify genes that could be the source of disease. The process typically takes less than two days to complete, even though the data equates to over a hundred gigabytes of information. In the second step, that mountain of material is processed and filtered by University of Utah experts in bioinformatics and then assessed by ARUP medical directors.
It’s time consuming, but “exciting, too, because we get more opportunities to identify some of the diseases we could not identify before,” Mao said. “Even when the outcome isn’t a happy one, we learn more and have more innovation.”
For some patients in the NICU, data analysis can begin before birth if doctors identify the possibility of a known or suspected genetic disorder, Mao reported. In Nevaeh’s case, a CDH diagnosis was made during an ultrasound 16 weeks into Kimberly’s pregnancy, which allowed the NeoSeq team to conduct a review of other CDH cases and known clinical phenotypes, or characteristics, associated with CDH.
Kimberly, who goes by Kimmy, was also born with CDH, as are roughly one in 2,500 to 3,000 babies each year. Depending on its severity, CDH can be a life-threatening genetic disorder. CDH leaves a hernia, or hole, in the diaphragm that allows the abdominal organs to move into the chest as the baby grows. That movement can impede the development of the lungs, causing a condition known as pulmonary hypoplasia, which results in breathing difficulties.
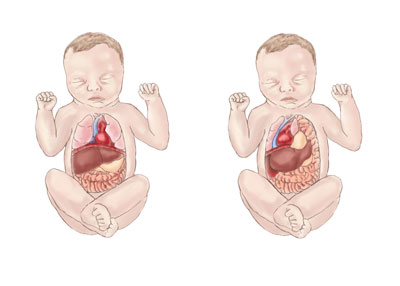
What is a congenital diaphragmatic hernia?
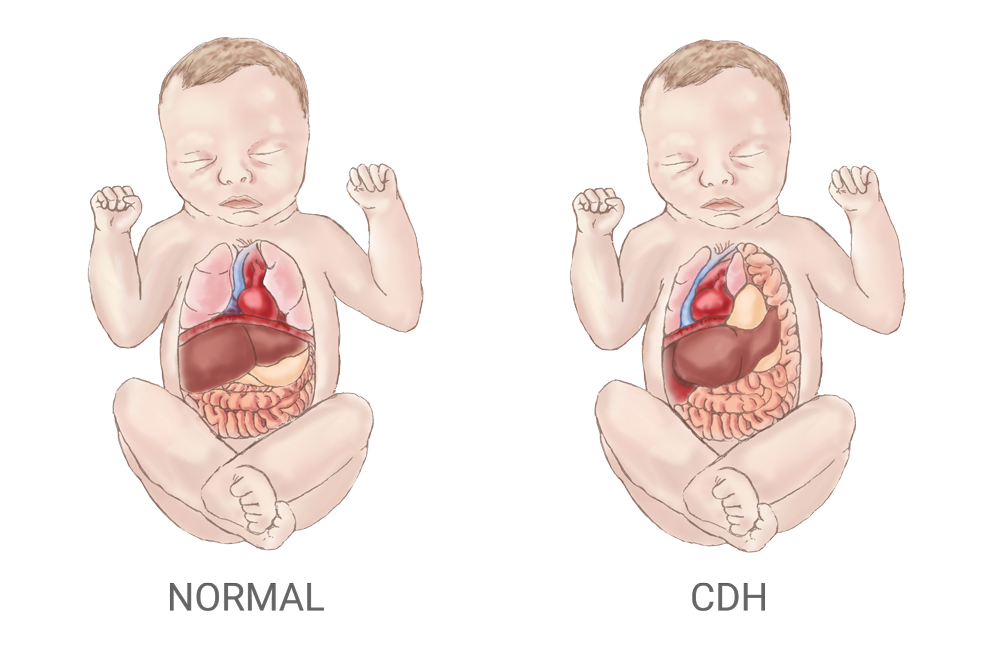
A congenital diaphragmatic hernia (CDH) leaves a hole in an infant’s diaphragm that allows the stomach, bowels, and liver to slip through and move into the chest, which crowds other organs and restricts the development of the lungs and heart. The drawing on the left shows the normal placement of organs in a child; on the right is an infant with CDH.
Kimberly’s case of CDH was mild. She has experienced breathing challenges throughout her life that have limited her physical activity, and she has relied on supplemental oxygen at times. Armed with this information, the NeoSeq team was able to streamline its analysis by focusing on genes that are known to cause this condition and comparing likely variants with Kimberly’s genome. Both mother and daughter were also born with an atrial septal defect (ASD), a defect characterized by a hole in the heart that divides its separate chambers. A surgery at age 5 fixed Kimberly’s ASD. Doctors have not yet addressed Nevaeh’s ASD and believe it may be relieving pulmonary pressure on her lungs, but surgery may be considered later.
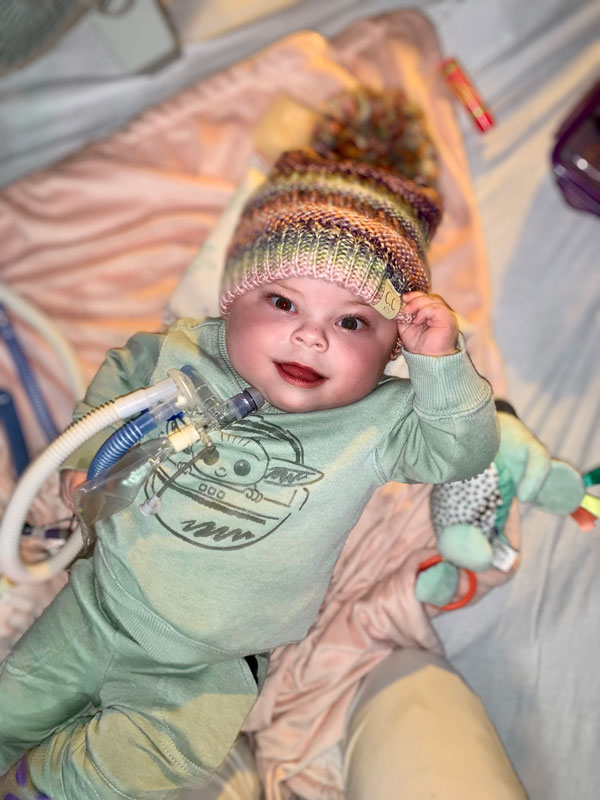
Nevaeh’s diagnosis was not really a surprise, but Kimberly said she was naive about the high likelihood (50%) of passing CDH on to her daughter, and she did not expect such an extreme presentation of the disease.
“I never thought I would have a baby with CDH,” she said. “But I guess in my gut, I was also like, ‘Why would I have a healthy baby?’”
She and her husband Chris said they opted to join the NeoSeq pilot project because they felt the study would provide them with useful answers, and not just for their daughter. Kimberly wanted a clearer picture of her own experience with the condition.
Nevaeh, who weighed just 3 pounds at birth, needed surgeons to build a diaphragm for her when she was 8 days old. Since then, she’s been on and off ventilators, been diagnosed with pulmonary hypertension, had surgery for a second diaphragmatic hernia when she was 4 months old, was exposed to COVID-19, and suffered a heart attack.
“None of it would have happened if she didn’t have CDH,” said Kimberly.
Doctors wouldn’t have been able to pinpoint Nevaeh’s condition and make a firm diagnosis without whole genome sequencing, according to Nevaeh’s doctor, Sabrina Malone-Jenkins, MD, an assistant professor of pediatrics at the U in the Division of Neonatology.
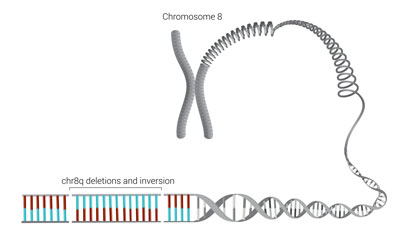
More than simply identifying some variation in a gene known to be associated with CDH, the laser-like detail of genome sequencing found two deletions and a complicated rotation called an inversion in chromosome 8, which essentially scrambled the code for that part of Nevaeh’s genome.
Chromosome 8
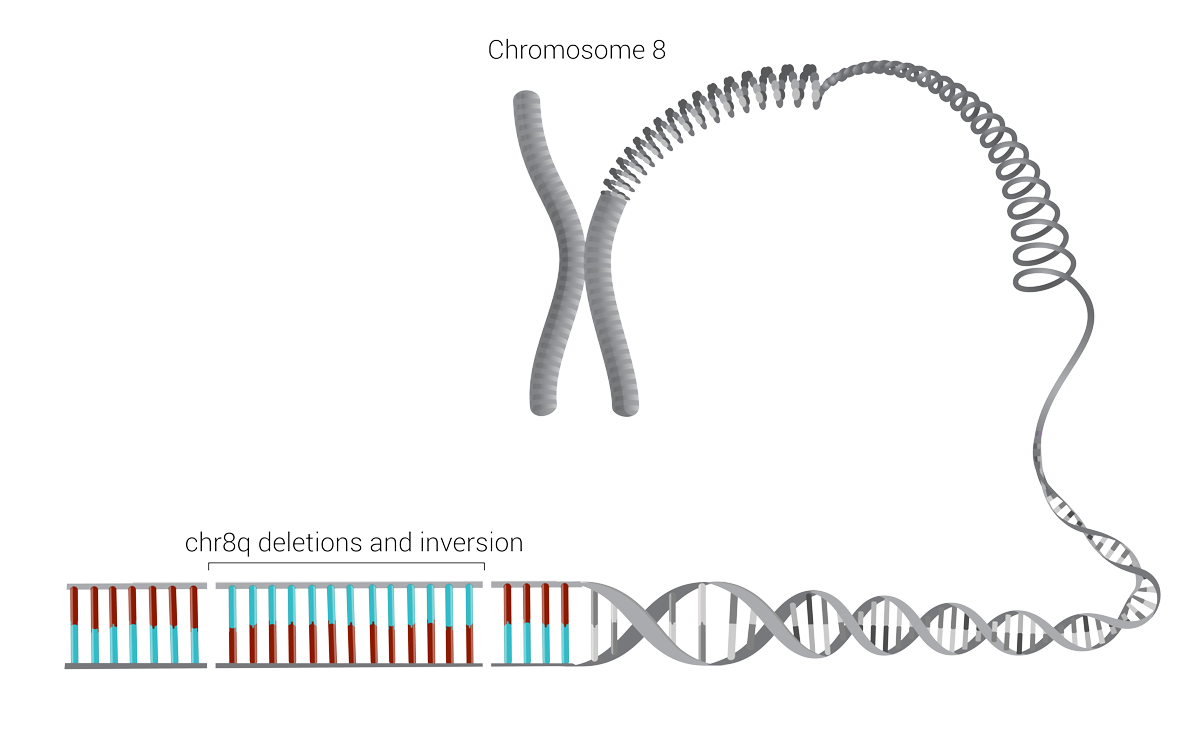
Chromosome 8 is associated with congenital diaphragmatic hernias. Nevaeh Pass’s severe form of the condition was caused by two deletions and an inversion within it, shown here as breaks in the continuous line of the chromosome and the reversal of the blue/red color patterns.
Kimberly’s genome showed the same pattern, which confirmed that the condition is inherited from the mother, Mao stated.
It’s a scientifically significant discovery made and confirmed by ARUP’s Cytogenetics team, one of the best in the country for identifying and confirming the presence of broken, rearranged, or missing chromosomes.
“This sort of complicated gene arrangement was not detectable with previous rapid sequencing,” Mao said.
Best explained, “That’s why ‘trio’ testing of parents and babies is both crucial and revolutionary.” Whole genome sequencing can reveal hundreds of thousands of alterations or changes in DNA. Using the data from parents helps filter out all the genetic information unrelated to a baby’s phenotype, or traits, and helps zero in on what is causing the condition.
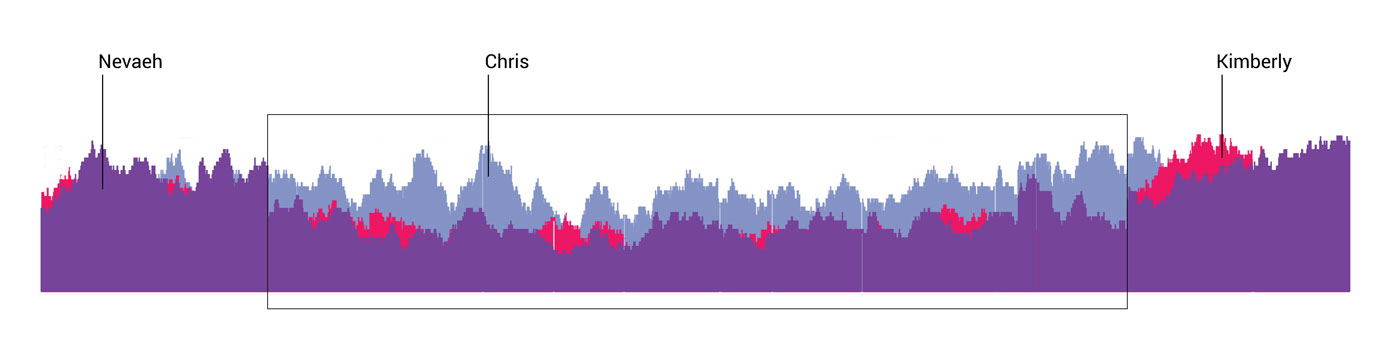
Trio testing
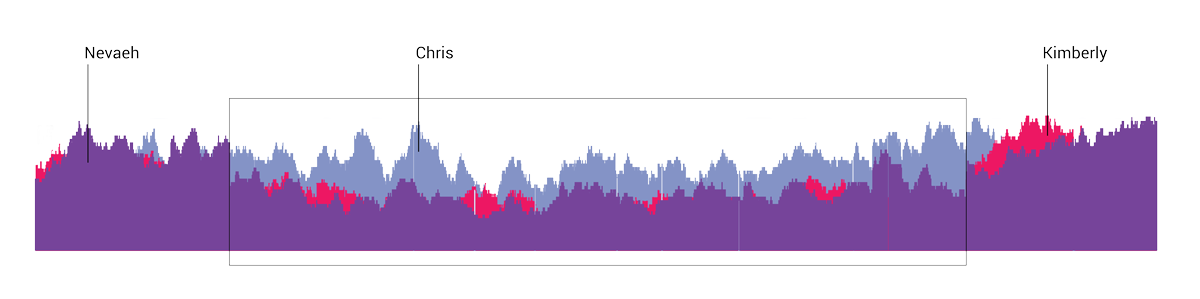
Trio sequencing compares the genome of both parents and their newborn so that doctors can identify differences and pinpoint the chromosomes that may be the source of a genetic condition or disease. In this diagram, Chris Pass’s genome is represented in blue, Kimberly’s is in red, and Nevaeh’s in purple. The differences confirmed that CDH is passed from mother to child.
This type of testing also aids in detecting “de novo” genetic variants that are not found in the parents and only found in the baby, which can help point to the source of the disease or condition.
“It’s the most inclusive type of testing that you can do, and it offers the benefit of not just what we can see now, but also a long-term benefit,” Malone-Jenkins said. “We don’t know the function of every gene or every variant, but as the science advances, we can continue to look back at the changes we were able to see and potentially find answers.”
Giving families the information they need to make informed choices is important but sometimes difficult. On the best day, having detailed genetic information means that doctors are able to identify a treatable disease early enough to provide treatment that can result in rapid improvements. Other times, genetic analysis can mean delivering the devastating news that a baby has a terminal condition.
“One of the most meaningful cases we’ve had in this process was the diagnosis of a kid who was not going to recover,” Best said. “It gave the family some closure and the support they needed to make the decision to withdraw [life] support. In the field of genetics, it’s a very important thing.”
Whole genome sequencing can also guide couples in making decisions about future pregnancies and help them understand what options might exist for prenatal or even preimplantation diagnosis or intervention.
“That’s something very powerful for Kimmy and her husband to know,” Malone-Jenkins said. “They would have a 50% chance of having another baby with this presentation.”
The Passes say they are still weighing those implications and are not sure they would want to see another child suffer a medical journey like Nevaeh’s.
Nevaeh’s case also shows what Malone-Jenkins called the “superiority” of the NeoSeq project’s analysis pipeline, its bioinformatics team, and the tools they are using.
“I couldn't even find another clinical laboratory that could validate these results because they're just not able to do that,” she said. ARUP developed an in-house validation test, and Malone-Jenkins explained, “ARUP and the U had the infrastructure in place to do this and were building out the processes and protocols to hopefully expand the availability of the testing to the entire Mountain West.”

Beyond CDH, Malone-Jenkins said, the NeoSeq project is also “moving the science forward” in diagnosing congenital heart defects and hydrops fetalis, and the project is working toward identifying several new functions of genetic variants.
ARUP has its sights set on using these lessons to make the rapid whole genome sequencing test available to clients in all 50 states, perhaps as soon mid-2022 when the pilot wraps up, Best stated.
“I think the whole genome sequencing is the best thing we could have done for Nevaeh,” Kimberly said. “It will give [doctors] more knowledge. Because, you know, one day a family is going to be at their 20-week ultrasound and hear the words congenital diaphragmatic hernia for the first time and they are not going to know what to do with that.”
There have been times, Kimberly said, when she has wondered if the whole journey has been too hard on their daughter, or if the medical responses to each new crisis were selfish choices for them to make. Still, Nevaeh has recovered each time like a trooper and continues to make improvements.
Remarkably, as of her birthday in January of 2022, Nevaeh no longer needed intravenous medications through a catheter and was being readied for an out-of-hospital ventilator so she could go home with her parents.
“She smiles, she plays, she giggles. It’s all worth it,” said Kimberly, who notes there are unknown hurdles, including surgeries, ahead.
Although she remains on an at-home ventilator, Nevaeh had recovered enough by mid-February that she was able to go home with her parents. It was a day they had often worried would never come.
“I don’t think it’s fully hit us,” said Kimberly. “We are excited, but also nervous. It doesn’t feel real.”