ARUP Continues to Pioneer Improved Drug Testing to Support Patient Care
Almost a decade ago, ARUP Laboratories launched a new drug panel that countered all known and accepted approaches to drug detection. The approach, pioneered by a visionary clinical toxicologist and ARUP medical director, Gwen McMillin, PhD, DABCC (CC, TC), FAACC, eliminated the traditional screening test that was followed by a confirmatory test.
Instead, McMillin proposed a new philosophy that would provide more accurate results in less time, reduce unnecessary testing, and decrease testing costs. The philosophy was simple: Use the test that best fits the clinical need, even if that means skipping the standard screening test.
“If you know that a screening test isn’t going to give an accurate result, doesn’t perform well, or doesn’t meet your clinical needs, then why do it at all?” McMillin said. “I knew that, in many cases, it would be better to bypass the screening test and go straight to the more sensitive, more accurate test.”
According to McMillin, immunoassays are often used as an initial screen because they can provide results quickly, they are inexpensive, and the equipment used to perform them is more readily accessible to clinical laboratories. Immunoassays are also known to cross-react with multiple compounds and therefore provide ambiguous results that require confirmation. By contrast, mass spectrometry testing can yield a very specific and accurate result.
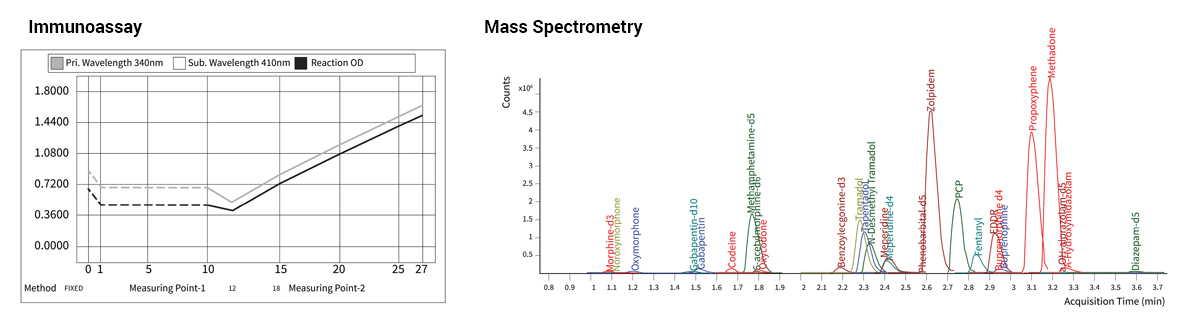
“The team has built a lean, targeted panel that provides the information clinicians need and reduces confusion regarding results,” said Jessica Boyd, PhD, FCACB, DABCC (TC), the medical director of Clinical Toxicology who now oversees the drug panel.
The new panel, the first for which this mass spectrometry-first approach was used, initially met with resistance. McMillin fielded call after call from other laboratorians and clinicians who found the strategy baffling.


“The approach was originally seen as somewhat clinically unorthodox,” said Gordon Nelson, the Chemistry operations director who participated in the initial launch. “Clinicians were so dialed in to the idea that you needed to confirm every single drug test that they didn’t understand the logic.”
Confident in the soundness of her philosophy, McMillin persevered, devoting significant effort to educating colleagues and clinicians and establishing the beneficial outcomes.
“We have centered our efforts around innovation and what makes sense for the patient,” McMillin said. “Many other labs have followed in our footsteps in trying to focus on what’s needed for clinical lab management.”
Now, 10 years later, many other laboratories have adopted McMillin’s once unconventional method, and McMillin has since helped to write new laboratory guidelines for drug testing based on this strategy to promote more efficient, effective clinical outcomes.
That determination to continuously drive change that leads to better patient outcomes is a characteristic common to all of the team members in ARUP’s Clinical Toxicology Department. Their efforts have transformed a single, small clinical laboratory with about 20 employees into a thriving, robust department—with about five clinical laboratories and more than 200 employees—that delivers quality results for tens of thousands of patients each month.
“We’ve tried to evolve in concert with our clients’ needs, and what patients and clinicians need, so that we can respond, even if that’s breaking down traditional approaches and models,” McMillin said.
This month, ARUP’s Clinical Toxicology Labs will complete their move into ARUP’s new state-of-the-art facility. The move marks the culmination of more than six years of effort to design a laboratory uniquely suited to mass spectrometry testing.
“We’ve carefully considered every aspect of this space—from how to limit the noise to make the lab staff more comfortable to using the excess heat the machines generate as a heat source for the building,” said Warren Hunt, Chemistry group manager, who has played a key role in designing the new space and making it operational.
The new lab will group testing into two categories, quantitative and qualitative mass spectrometry, and increase both operational capacity and efficiency.
“We’ve consolidated our processes to streamline tests that are performed on the same types of instrumentation and that generate the same types of results, aligning inputs to outputs,” McMillin said.
This isn’t the first time the Clinical Toxicology Labs have successfully combined testing in such a way that it resulted in big wins. Several years ago, as Clinical Toxicology shifted its focus to mass spectrometry testing on the heels of McMillin’s revolutionary approach, the lab moved all immunoassay testing to ARUP’s Automated Core Lab.
“That consolidation really allowed the Automated Core Lab to focus on what they do best, and the Clinical Toxicology Labs to focus on our strength, which is mass spectrometry,” Nelson said.
As a result, the turnaround time for immunoassays decreased by 30%, and the capacity for mass spectrometry tests more than doubled. The team hopes the current consolidation will further increase efficiency and capacity.
“In toxicology, whether you’re screening for drugs of abuse or drugs that are prescribed, speed and accuracy are the two keys that drive operations, because there’s always someone who is waiting on a dosing adjustment or a treatment plan,” Hunt said.
Building Tests That Support Patient Care
Building efficient testing processes has lasting effects well beyond the laboratory. For clinicians who need information, sometimes urgently, to begin effective treatment, efficient testing can mean the difference between successful treatment and failure.

In 2022, Kamisha Johnson-Davis, PhD, DABCC (CC, TC), medical director of Clinical Toxicology, launched a new expanded drug profile that supports emergency departments across the nation in cases of unknown drug exposure. The profile detects an impressive 127 drugs and drug metabolites, including prescription, over-the-counter, and illicit drugs. For patients who are experiencing adverse reactions to an unknown drug, identifying the cause quickly and accurately is critical to starting effective treatment.
“You can imagine the scenario of a pediatric patient who has unknowingly gotten into the medicine cabinet and is experiencing an adverse reaction to that exposure,“ Johnson-Davis said. “It’s critical that we determine quickly what that drug is so that the patient can begin receiving a potentially lifesaving treatment.”
As part of her efforts, Johnson-Davis consolidated the test onto one platform, which makes it possible for the test to detect all 127 drugs with a single injection. According to Johnson-Davis, consolidating testing onto one platform not only increases efficiency, but it also enables better testing of pediatric samples.
“We no longer have to split a sample between multiple platforms, which has significantly reduced the amount of sample required,” Johnson-Davis said. “It is often difficult to collect sufficient quantities from pediatric patients. With the single injection, we can run the test from even just 1 milliliter of sample.”
Johnson-Davis carefully selected which drugs to include in the panel based on data from the American Association of Poison Control Centers on the most common accidental exposures.
“It’s impossible to include everything because there are hundreds and hundreds of drugs,” Johnson-Davis said. “I wanted to build a test that would support the top clinical needs. We designed the test to pick up routine medications that could be found at home.”
“We were one of the first laboratories to go live with a broad-spectrum screen by mass spectrometry; now we have a mass spectrometry test that covers 127 different compounds with a single injection,” said Hunt, the Chemistry group manager. “It’s truly remarkable when you think about the amount of progress that has occurred in a relatively short time span.”
Individual, Precise Diagnostic Medicine

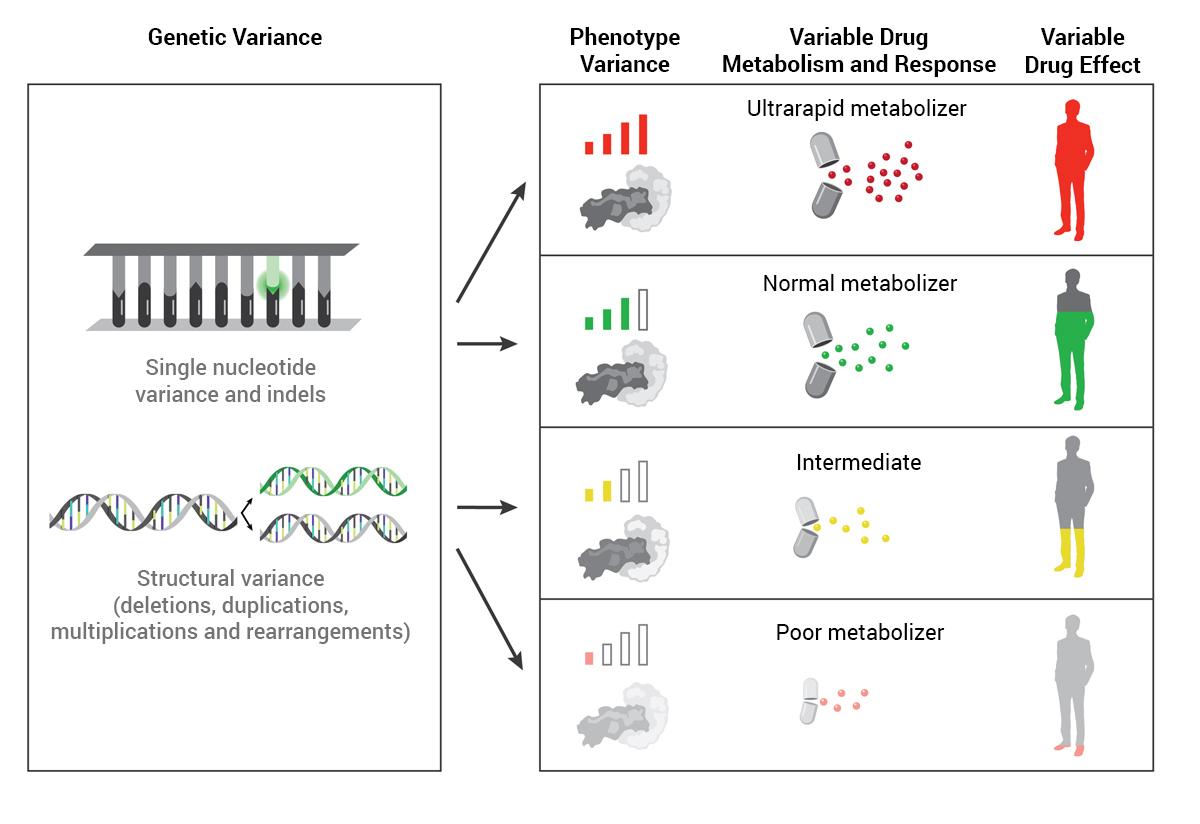
Pharmacogenomics (PGx), an emerging medical specialty, leverages genetic testing to predict how patients are likely to respond to certain medications. PGx testing aims to select the most appropriate drug options and doses for individual patients and avoid adverse drug effects.
“Drug manufacturers base their dosing on what is most appropriate for the vast majority of the population,” said Ryan Nelson, PharmD, medical director of Precision Medicine. “The challenge with that approach is that not everybody is the same, and we all have slight variations in how we react to or metabolize drugs.”
According to Nelson, a recent study involving members of the Kentucky Teachers Retirement System found that using PGx resulted in a 15% reduction in inpatient visits, a 6.8% reduction in emergency department visits, and a cumulative savings of $37 million during a 32-month period.
One issue hindering the implementation of PGx in the clinic is a lack of consensus between regulatory and guideline-producing authorities on when and how PGx should be used.
Nelson is currently working to build an open-source database, MetaCensus, using blockchain technology, that will make relevant clinical information on PGx free for anyone to access. Additional scientific domains will be added to MetaCensus as it continues to grow.
“Most clinicians cannot afford to access the most recent data published on PGx,” Nelson said. “This hinders clinicians worldwide from accessing reliable information promptly and, in turn, hinders them from providing the best care based on the most recent information in the field.”
Nelson hopes this open-source database will enable clinicians to access up-to-date information in a concise format so that they can make better-informed clinical decisions, and that it will change how meta-analyses are performed. He has gathered a team of topic experts in precision medicine and data science to volunteer their time to build MetaCensus.
“We’ve seen significant enthusiasm from the PGx community members, who are volunteering their time to build MetaCensus to accelerate scientific consensus and produce more robust meta-analyses,” Nelson said.
MetaCensus will function as a central resource for industry and medical experts to review and assess the same data, then determine how to interpret and apply the evidence.

Sherin Shaaban, MD, PhD, FACMG, medical director of Pharmacogenomics and Molecular Genetics, has led the development of PGx testing. Shaaban has carefully curated the genes included in ARUP’s PGx panels based on research that has demonstrated the clinical relevance of those genes to drug metabolism.
“It’s important that we offer testing for genes and variants that are actionable and have the highest levels of evidence of their role in drug metabolization,” Shaaban said.
According to Shaaban, many healthcare providers may still be reluctant to order PGx testing because they do not feel comfortable choosing the test and interpreting the results. Shaaban and her colleagues are available to consult with clinicians on test ordering and results interpretation.
“The interpretation, especially for the two larger panels, can become complicated,” Shaaban said. “We want to help physicians navigate the complex world of pharmacogenomics.”
Recently, Shaaban consulted with a physician who had contacted ARUP about one of his patients, a child with autism who had been on a treatment plan that effectively managed his condition for a long time, but whose condition then started to deteriorate. Shaaban recommended the physician order a psychotropic panel.
“The physician reported back a few months later that they had needed to change a lot of dosing and drug choices, but the new treatment plans—based on the patient’s genetic ability to metabolize drugs—had helped to stabilize the patient’s symptoms,” Shaaban said.
“I’m proud of the quality of testing we offer. It’s an additional layer of patient care that makes it possible to individualize the management of patients from drug choice to proper dosing,” Shaaban said. “We’ve focused our strategy on providing results that will actually be useful to clinicians as they make decisions for their patients.”











