Most Popular
April 29, 2024ARUP maintains that the FDA’s rule will limit access to testing, stifle innovation, and increase healthcare costs.
November 29, 2023ARUP believes the FDA’s plan to regulate lab-developed tests will limit access to testing, increase costs, and face a court challenge, and is calling for collaboration to better address LDT oversight.
March 20, 2024ARUP’s new test, Alzheimer’s Disease Markers, CSF, will pave the way for more assays that help detect the disease soon enough to try therapy to slow its progression.
April 26, 2024Nicola Longo, who with his wife, Marzia Pasquali, is credited with expanding screening for a treatable metabolic disorder, is leaving for UCLA while Pasquali stays at ARUP to continue key projects.
July 12, 2024ARUP’s chief medical officer urged the House Committee on Ways and Means to consider oversight structures that both protect the public health and support innovation in healthcare.
May 30, 2024
ARUP Files Declaration to Support Lawsuit Challenging the FDA's Rule to Regulate Lab-Developed Tests
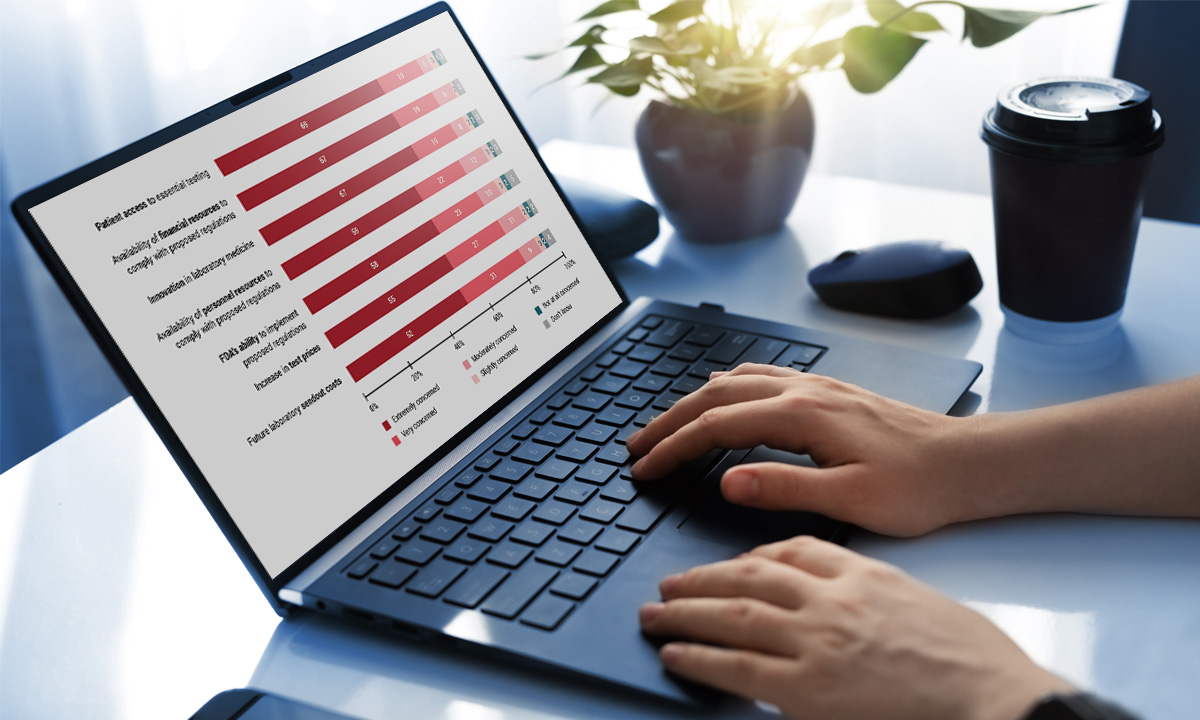
ARUP’s declaration amplifies the lawsuit’s claims that the FDA does not have legal authority over laboratory testing services and that regulating these services is unreasonable.March 5, 2024Nearly 85% of respondents to an ARUP survey believe the FDA’s proposed rule to regulate lab-developed tests will negatively impact their labs. Only 3% believe they have the financial resources to
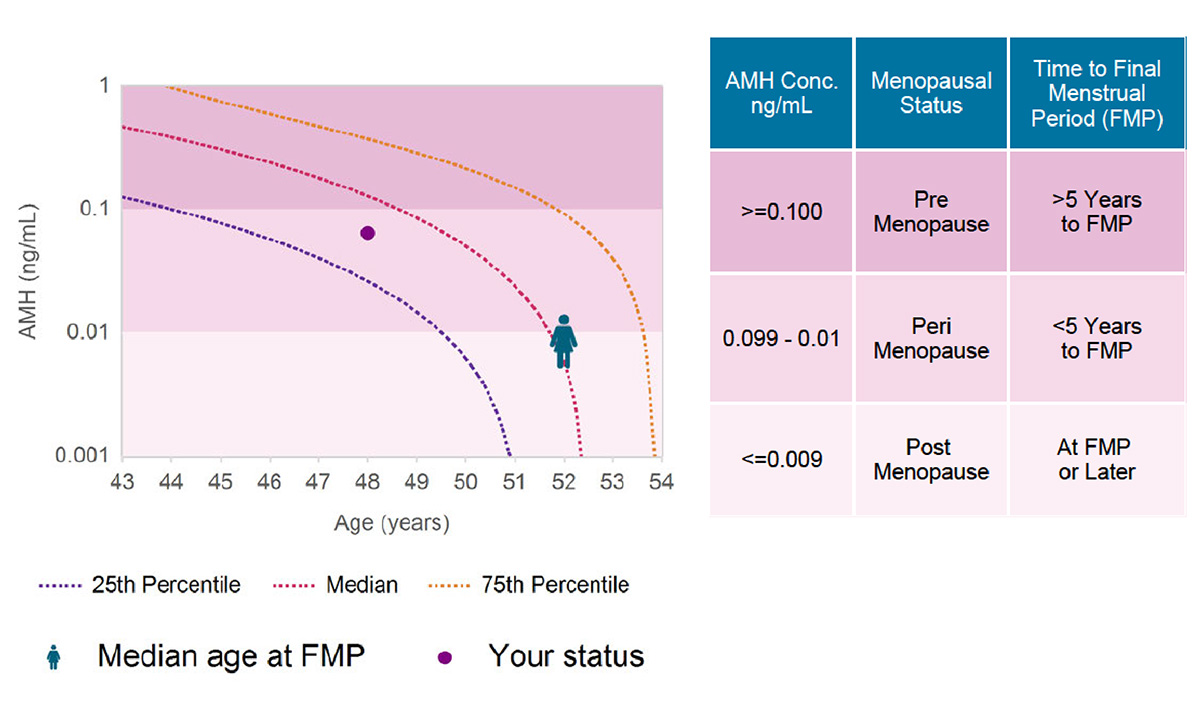
February 22, 2024The MenoCheck test helps providers treat women who are experiencing menopausal transition and manage the health risks associated with decreased estrogen production.
April 24, 2024A new partnership between ARUP and connectivity provider ELLKAY aims to give health systems the testing support, guidance, and technology needed to establish or expand lab outreach operations.
August 27, 2024
ARUP Medical Laboratory Scientist Celebrates More Than 40 Years With University of Utah Hospital Lab
Susan Driggs, a medical laboratory scientist in ARUP’s University of Utah Hospital Clinical Laboratory, has worked in the same lab since before ARUP’s inception in 1984.November 10, 2023
EU Approval of AAV5 DetectCDx™ Provides Access to Companion Diagnostic for Hemophilia A Gene Therapy
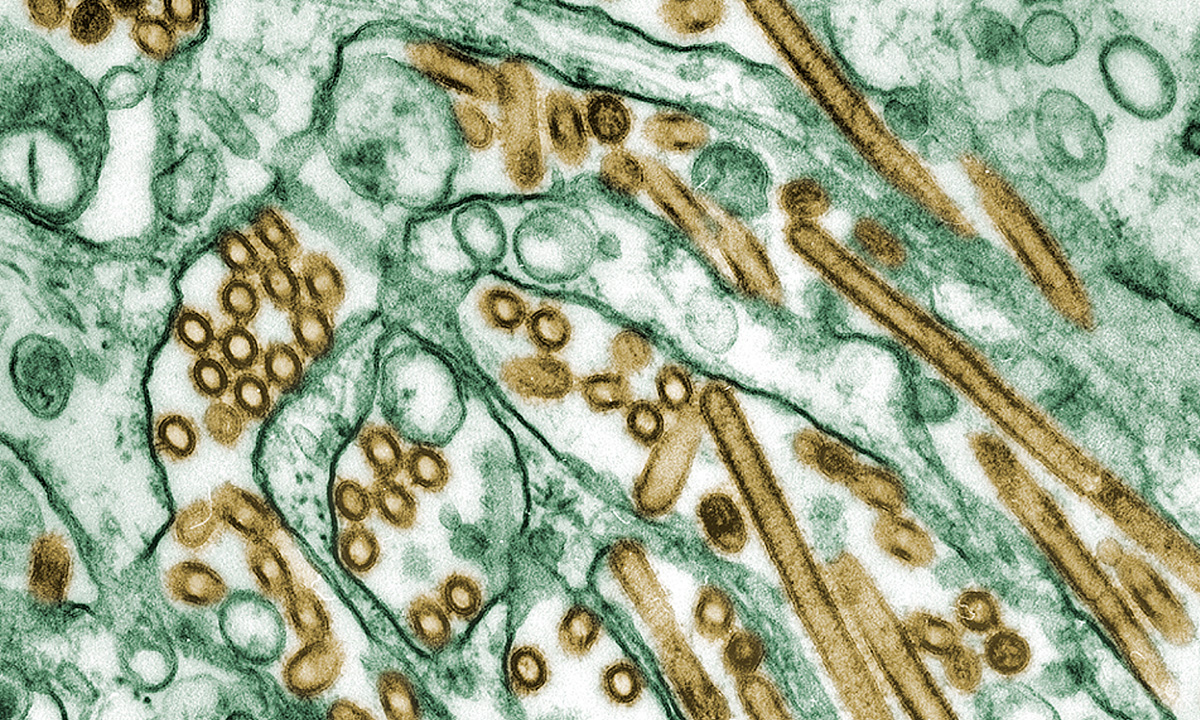
ARUP has gained a Conformité Européenne (CE) mark for AAV5 DetectCDx™ single-site use. The test will aid in determining the eligibility of non-U.S. patients for a new hemophilia A gene therapy.September 13, 2024ARUP has been awarded a CDC contract for avian influenza A (H5N1) test development, which recognizes our expertise and experience with assay development and our desire to serve public health needs.
July 8, 2024ARUP Laboratories has been chosen as the site for a House Ways and Means Committee Field Hearing on July 12. Topics include medical innovation, healthcare access, and economic prosperity.
April 19, 2024Jan Wintch, CQA(ASQ), senior quality specialist, has worked at ARUP since the company’s inception. She enjoys her current role in Quality and remembers ARUP’s early years fondly.
January 10, 2024After wrapping up her education in plant biology, Dipanwita Banerjee, MS, took some time off to see the world and start a family. She later returned to the laboratory environment at ARUP.
December 1, 2023Utah Business magazine honored ARUP Laboratories with its Best Companies to Work For award for the sixth consecutive year based on anonymous employee survey results.
May 28, 2024ARUP Laboratories is committed to helping clients and the clinical laboratory community navigate regulatory changes and is offering current resources and a new webinar.
December 6, 2023ARUP and Medicover have partnered to provide a new companion diagnostic test to European Union patients. The test helps identify individuals eligible for a new gene therapy for severe hemophilia A.