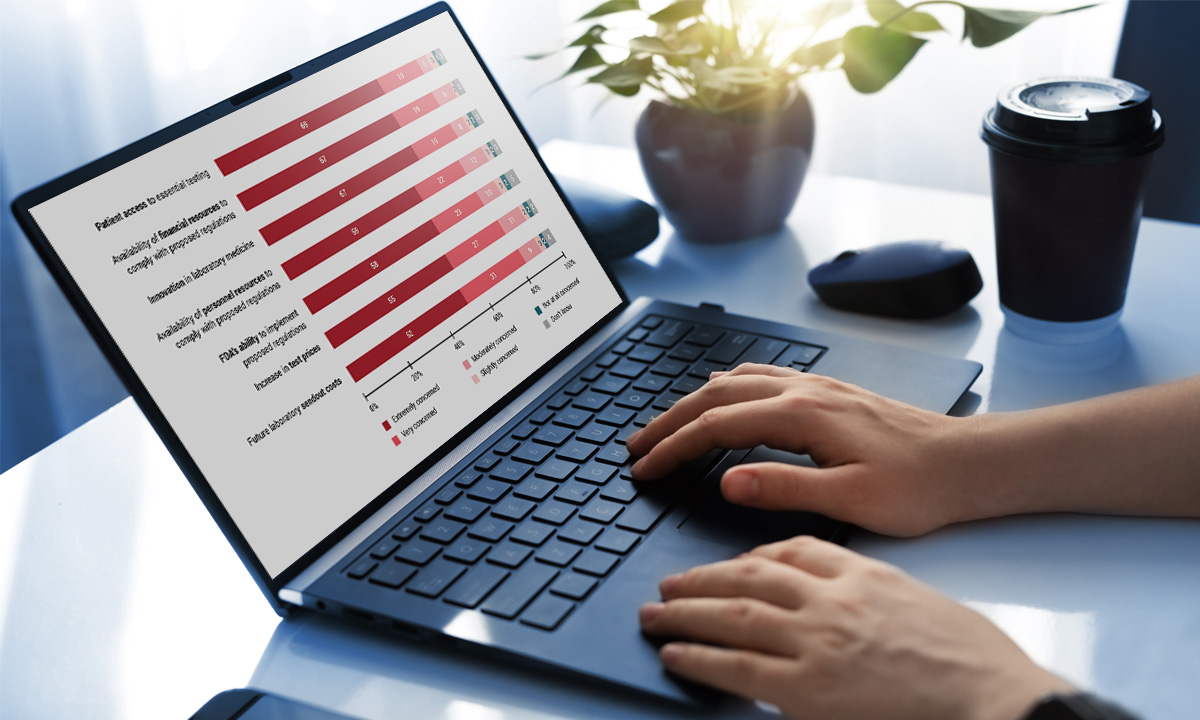
Nearly 85% of respondents to an ARUP survey believe the FDA’s proposed rule to regulate lab-developed tests will negatively impact their labs. Only 3% believe they have the financial resources to
ARUP experts seek to increase understanding of the FDA’s proposed rule to regulate lab-developed tests as medical devices and the impact on hospitals and patients during a webinar on November 29.
A platform clients use to reduce testing waste, lower cost per test, and improve patient safety has won the prestigious Choosing Wisely Champion Award from the American Society for Clinical Pathology.
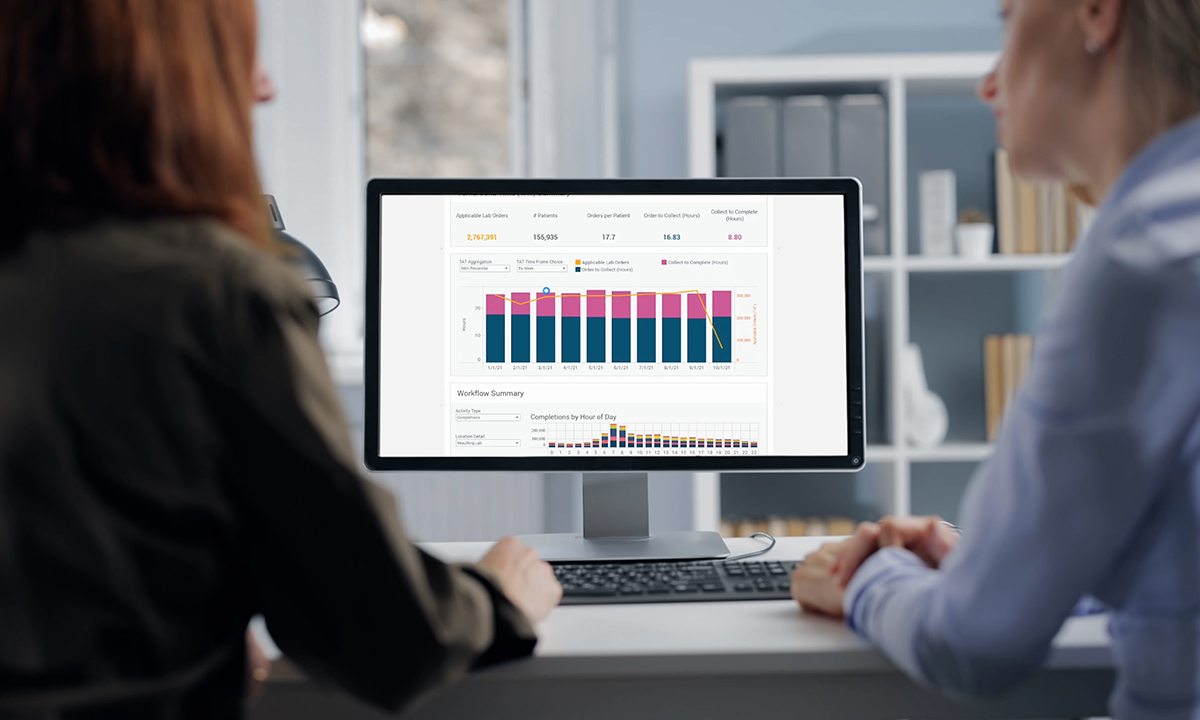
ARUP’s new tool, AnalyticsDx™ Comprehensive, provides cohesive data visualization that clients can use to inform quality improvements and cost-saving measures.
Data from the 2022 Client Satisfaction Survey shows that an overwhelming majority of our clients view ARUP favorably and would recommend ARUP to colleagues.
For 20-plus years, Mark Withers had inexplicable episodes of a severe anaphylaxis-like phenomenon. ARUP’s unique expertise and highly sensitive tests help patients like him find the right diagnosis.
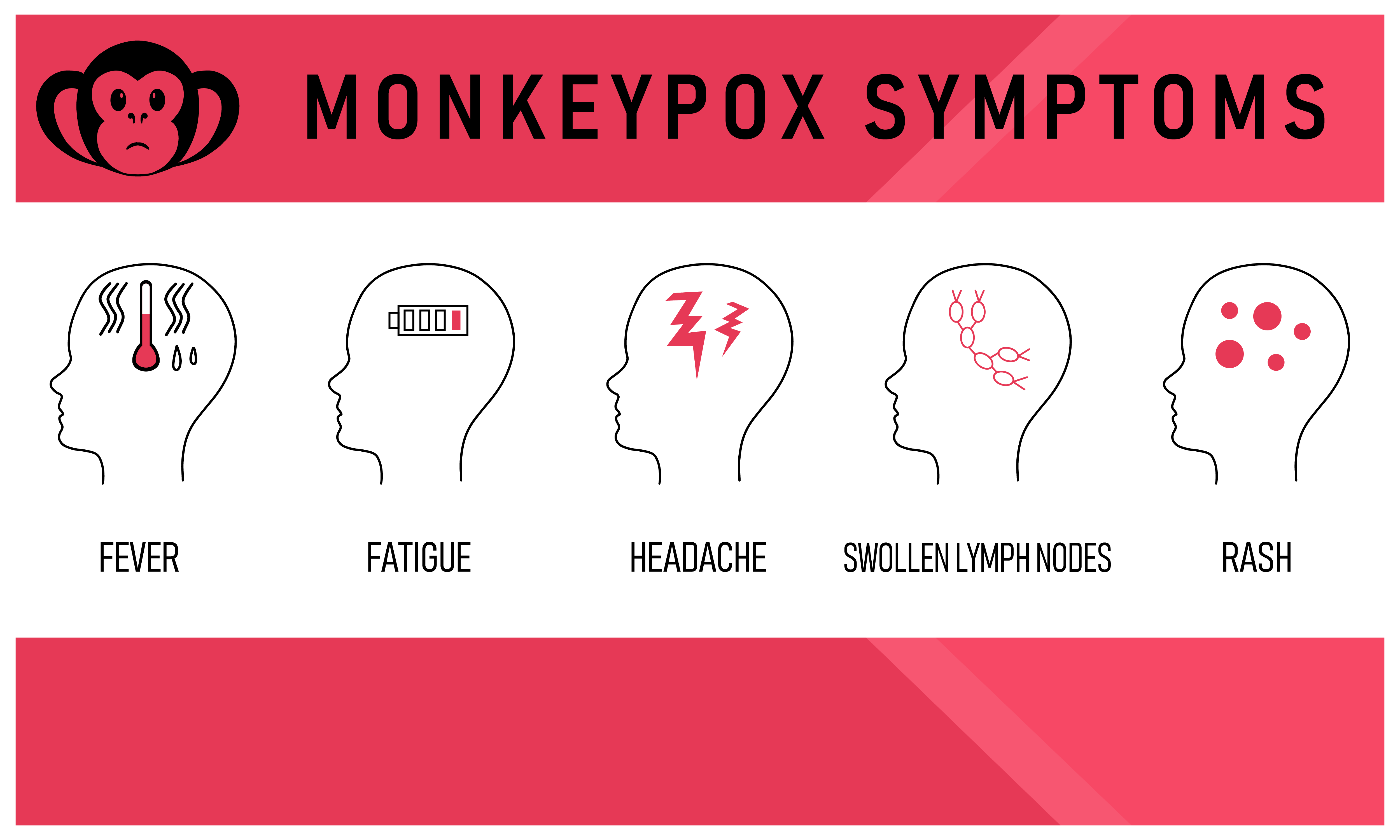
As of May 23, multiple cases of monkeypox, a rare zoonotic infection endemic to Central and West African countries, had either been confirmed or were being investigated in at least six U.S. states.
On December 10, 2021, a Common Vulnerability and Exposure (CVE) alert was issued from NIST related to Apache Log4j, CVE-2021-44228.
With ARUP Connect™, clients can access their test orders and records, order supplies, and otherwise interact online easily with ARUP regardless of where they are, using a simple laptop and password.