Test Highlight: New CMV Test Live November 14, 2022
| Test Code | Test Name |
|---|---|
| 3005895 | Cytomegalovirus by Quantitative NAAT, Plasma |
One of the most common complications following an organ transplant is cytomegalovirus (CMV) infection.1 Nucleic acid amplification testing (NAAT) methods such as polymerase chain reaction (PCR) detect and quantify the viral load in a specimen and thus are vital in the diagnosis and management of CMV infections. Quantitative molecular testing is also useful in monitoring a patient’s response to treatment and predicting clinical progression to disease or clinical relapse.1
To assist in the detection and surveillance of CMV infections, ARUP now offers a U.S. Food and Drug Administration (FDA)-cleared assay for the quantification of CMV DNA in plasma specimens.
The benefits of the new test, Cytomegalovirus by Quantitative NAAT, Plasma, include:
- A 1–2-day turnaround time (TAT) will ensure that patients get their results sooner and can begin treatment earlier. A rapid TAT is especially important for immunocompromised patients and patients receiving transplants.
- The test has a reproducible CMV quantitation at lower viral titers to provide the best likelihood of early and accurate detection.
- The test’s broader quantitative range will provide physicians with relevant information regarding the patient’s viral load.
- This FDA-cleared assay supports the standardization of CMV results across the laboratory industry to optimize patient treatment.
- Test transition to FDA-cleared assays supports more stringent vendor quality and corresponds with ARUP’s overall quality plan.
- Testing is performed on a single specimen type, which is preferred for patient monitoring.
- This test is FDA-cleared, which means it will be easier to bill to Medicare. Clients will no longer need to submit an additional code on claims to be reimbursed for this testing, and insurance reimbursement is more consistent for FDA-approved tests.
Frequently Asked Questions
What are the differences between the inactivated 0051813 Cytomegalovirus by Quantitative PCR and the new 3005895 Cytomegalovirus by Quantitative NAAT, Plasma test?
|
|
0051813 Cytomegalovirus by Quantitative PCR |
3005895 Cytomegalovirus by Quantitative NAAT, Plasma |
|
Reference Range |
227–2,270,000 IU/mL 2.4 log IU–6.4 log IU |
35–10,000,000 IU/mL 1.54 log–7.00 log IU/mL |
|
TAT |
1–3 days |
1–2 days |
|
Sample Types |
Plasma, whole blood, or bronchoalveolar lavage |
Plasma |
|
Sample Stability |
Ambient: 24 hrs |
Ambient: unacceptable |
|
Category |
LDT |
FDA cleared |
|
Sample Volume |
1 mL |
2 mL |
Which CMV genotypes can be quantified using the Cytomegalovirus by Quantitative NAAT, Plasma test?
This test can quantify CMV DNA from glycoprotein B genotype 1–4.2
Which test should I order if I would like to submit a whole blood or bronchoalveolar lavage (BAL) sample?
I am tracking my patient’s viral load, do I need to reestablish a baseline?
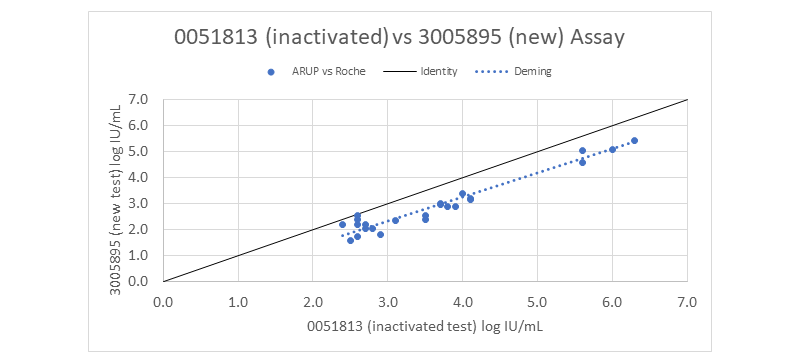
Based on verification studies, the inactivated laboratory-developed test (0051813 Cytomegalovirus by Quantitative PCR) was approximately 0.7 log IU/mL higher than the new FDA-cleared test (3005895 Cytomegalovirus by Quantitative NAAT, Plasma) performed on the Roche platform.
References
- Razonable RR, et al. Role of the laboratory in diagnosis and management of cytomegalovirus infection in hematopoietic stem cell and solid-organ transplant recipients. J Clin Microbiol. 2002;40(3):746–52.
- Roche Diagnostics. cobas® CMV. www.diagnostics.roche.com/global/en/products/params/cobas-cmv.html (accessed on October 11, 2022).